In the early stage of the outbreak, due to the rapid development, rapid diagnosis of suspected patients is the key to preventing COVID-19. Some approved nucleic acid detection reagents have a short development time, and there are problems such as hurried performance confirmation, insufficient reagent optimization, and large differences between batches; The problems of various clinical laboratories in various aspects of the nucleic acid detection process may also affect the accuracy of nucleic acid detection results. This article will focus on the key links and points in the current SARS-CoV-2 nucleic acid detection, and analyze the problems of false negative and positive re-examination of laboratory nucleic acid detection and clinical inconsistency.
Principles of SARS-CoV-2 nucleic acid detection
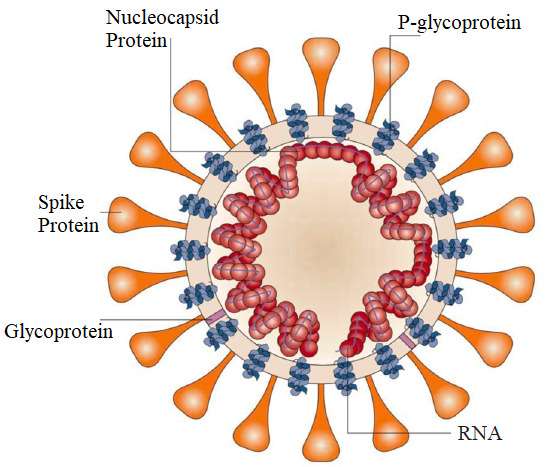
SARS-CoV-2 is an RNA virus with a genome sequence of about 29 kb, with 10 genes, which can effectively encode 10 proteins. Viruses are composed of RNA and protein, and the outermost layer is an outer coating composed of lipids and glycoproteins. Inside, the protein capsid wraps the RNA in it, thereby protecting the easily degradable RNA (P1).
P1 Structure of SARS-COV-2
Viruses invade cells through specific cell surface receptors to cause infection, and use host cells to replicate.
The principle of viral nucleic acid detection is to expose the viral RNA through a cell lysate, and then use real-time fluorescent reverse transcription-polymerase chain reaction (RT-PCR) for detection.
The key to the detection principle is to use primers and probes to achieve “targeted matching” of nucleic acid sequences, that is, to find the nucleic acid sequence of SARS-CoV-2 that is different from other viruses in about 30,000 bases (the similarity of nucleic acid to other viruses) “Low” area), design primers and probes.
The primers and probes are highly matched with the specific region of SARS-CoV-2 nucleic acid, that is, the specificity is very strong. Once the real-time fluorescent RT-PCR amplification result of the sample to be tested is positive, it proves that SARS-CoV-2 is present in the sample. See P2.
P2 Steps of SARS-CoV-2 nucleic acid determination (real-time fluorescent RT-PCR)
Conditions and requirements of laboratory for SARS-CoV-2 nucleic acid detection
Nucleic acid testing laboratories are the most ideal for negative pressure environments, and they should pay attention to pressure monitoring, keep air flowing, and eliminate aerosols. Nucleic acid testing personnel must have corresponding qualifications, receive relevant polymerase chain reaction training and pass the assessment. The laboratory should be strictly managed, zoned in place, and irrelevant personnel are strictly prohibited from entering. The clean area should be ventilated and disinfected in place. Relevant items are placed in zones, clean and dirty are separated, replaced on time, and decontaminated in place. Routine disinfection: Chlorine-containing disinfectant is the main solution for larger areas, and 75% alcohol can be used for small areas. A good way to deal with aerosols is to open windows for ventilation, and air disinfection can also be carried out by means of ultraviolet rays, filtration, and air disinfection.
Key links and parameters of SARS-CoV-2 nucleic acid determination (real-time fluorescent RT-PCR)
Although laboratories generally pay close attention to nucleic acid “detection”, in fact, nucleic acid “extraction” is also one of the key steps for successful detection, which is closely related to the collection and storage of virus samples.
At present, the most widely used respiratory samples, such as nasopharyngeal swabs, use the second method, which is an inactivation (preservation) solution prepared based on nucleic acid extraction and lysis solution. On the one hand, this virus preservation solution can denature the protein of the virus, lose its activity and no longer be infectious, and improve the safety of the transportation and detection stage; on the other hand, it can directly crack the virus to release the nucleic acid, eliminate the nucleic acid decomposing enzyme, and prevent the virus. The RNA is degraded.
A virus sampling solution prepared on the basis of nucleic acid extraction lysis solution. The main components are balanced salts, ethylenediaminetetraacetic acid chelating agent, guanidine salt (guanidine isothiocyanate, guanidine hydrochloride, etc.), anionic surfactant (dodecane) Sodium sulfate), cationic surfactant (tetradecyl trimethyl ammonium oxalate), phenol, 8-hydroxyquinoline, dithiothreitol, proteinase K and other several or more components. At present, there are many types of nucleic acid extraction kits, and different nucleic acid extraction and purification reagents are used. Even if the same nucleic acid extraction and purification reagent is used, the extraction procedures of each kit are different.
At present, the nucleic acid detection kit products approved by the National Medical Products Administration are selected based on the ORF1ab, E and N genes in the SARS-CoV-2 genome. The detection principles of different products are basically the same, but their primers and probe designs are different. There are single-target segments (ORF1ab), dual-target segments (ORF1ab, N or E), and three-target segments (ORF1ab, N and E). The difference between detection and interpretation, nucleic acid extraction and real-time fluorescent RT-PCR reaction system should refer to the relevant kit instructions, and it is recommended that users strictly follow the interpretation method specified in the kit instructions for interpretation. The common regions, primers and probe sequences amplified by real-time fluorescent RT-PCR are shown in P3.
P3 The location of the SARS-CoV-2 amplicon target on the genome and the sequence of primers and probes
Interpretation of the results of SARS-CoV-2 nucleic acid determination (Real-Time fluorescent RT-PCR)
“Pneumonia Prevention and Control Plan for SARS-CoV-2 Infection (Second Edition)” for the first time clarified the criteria for judging the results of single gene amplification:
1. No Ct or Ct≥40 is negative;
2. Ct<37 is positive;
3. The Ct value of 37-40 is the gray-scale area. It is recommended to repeat the experiment. If the result of redoing Ct<40 and the amplification curve has obvious peaks, the sample is judged as positive, otherwise it is negative.”
The third edition of the guide and the fourth edition of the guide continued the above criteria. However, due to the different targets used in commercial kits, the aforementioned 3rd edition of the guide did not give the criteria for determining the combination of targets, emphasizing The instructions provided by the manufacturer shall prevail. Starting from the fifth edition of the guidelines, two targets have been clarified, especially the judgment criteria for a single target that is difficult to judge. That is, if the laboratory wants to confirm that a case is positive for SARS-CoV-2 nucleic acid detection, the following needs to be met 1 of 2 conditions:
(1) Two targets of SARS-CoV-2 (ORF1ab, N) in the same sample are tested positive by real-time fluorescent RT-PCR. If a single target is positive, re-sampling and re-testing are required. If the test results are If the single target is still positive, it is judged as positive.
(2) Two samples of real-time fluorescent RT-PCR showed a single target positive at the same time or two samples of the same type showed a single target positive test result, which can be judged as positive. However, the guidelines also emphasize that the negative results of nucleic acid testing cannot exclude SARS-CoV-2 infection. Factors that may cause false negatives need to be excluded, including poor sample quality (respiratory samples from oropharynx and other parts), sample collection too early or too late, Samples were not stored, transported, and processed correctly, and the technology itself had problems (virus variation, PCR inhibition), etc.
Causes of false negatives in the SARS-CoV-2 detection
The concept of “false negative” in nucleic acid testing that is currently concerned, often refers to “false negatives” in which the nucleic acid test results are inconsistent with clinical manifestations, that is, clinical symptoms and imaging results are highly suspected of COVID-19, but nucleic acid tests are always “negative” many times. The Clinical Laboratory Center of the National Health Commission explained the “false negative” SARS-CoV-2 test.
(1) There is a certain amount of virus in the cells of the infected person. Existing data show that after the body is infected by the virus, the virus enters the throat through the nose and mouth, then to the trachea and bronchi, and then reaches the alveoli. The infected person will experience the incubation period, mild symptoms, and then the process of severe symptoms, and different stages of the disease. And the amount of virus present in different parts of the body is different.
In terms of the viral load of cell types, alveolar epithelial cells (lower respiratory tract)> airway epithelial cells (upper respiratory tract)> fibroblasts, endothelial cells, and macrophages, etc.; from the sample type, alveolar lavage fluid (the most Excellent)>deep coughing sputum>nasopharyngeal swab>oropharyngeal swab>blood. In addition, the virus can also be detected in feces. However, considering the convenience of operation and the acceptance of patients, the commonly used clinical sample order is oropharyngeal swab>nasopharyngeal swab>bronchial lavage fluid (complex operation) and deep sputum (usually dry cough, difficult to obtain) .
Therefore, the amount of virus in the cells of the oropharynx or nasopharynx of some patients is small or extremely low. If only samples of the oropharynx or nasopharynx are taken for testing, the viral nucleic acid will not be detected.
(2) No virus-containing cells were collected during sample collection, or viral nucleic acid was not effectively preserved.
[① Improper collection site, for example, when collecting oropharyngeal swabs, the collection depth is not enough, the collected nasopharyngeal swabs are not collected deep in the nasal cavity, etc. Most of the cells collected may be virus-free cells;
②Sampling swabs are used incorrectly. For example, synthetic fibers such as PE fiber, polyester fiber and polypropylene fiber are recommended for the material of the swab head. Natural fibers such as cotton are used in actual operation (strong adsorption of protein and not easy to wash out) And nylon fibers (poor water absorption, leading to insufficient sampling volume);
③Incorrect use of virus storage tubes, such as misuse of polypropylene or polyethylene plastic storage tubes that are easy to absorb nucleic acids (DNA/RNA), resulting in a decrease in the concentration of nucleic acid in the storage solution. In practice, it is recommended to use polyethylene-propylene polymer plastic and some specially treated polypropylene plastic containers to store viral nucleic acids. ]
[① Improper collection site, for example, when collecting oropharyngeal swabs, the collection depth is not enough, the collected nasopharyngeal swabs are not collected deep in the nasal cavity, etc. Most of the cells collected may be virus-free cells;
②Sampling swabs are used incorrectly. For example, synthetic fibers such as PE fiber, polyester fiber and polypropylene fiber are recommended for the material of the swab head. Natural fibers such as cotton are used in actual operation (strong adsorption of protein and not easy to wash out) And nylon fibers (poor water absorption, leading to insufficient sampling volume);
③Incorrect use of virus storage tubes, such as misuse of polypropylene or polyethylene plastic storage tubes that are easy to absorb nucleic acids (DNA/RNA), resulting in a decrease in the concentration of nucleic acid in the storage solution. In practice, it is recommended to use polyethylene-propylene polymer plastic and some specially treated polypropylene plastic containers to store viral nucleic acids. ]
(4) The clinical laboratory operation is not standardized. Sample transportation and storage conditions, standardized operation of clinical laboratories, result interpretation and quality control are the key factors to ensure the accuracy and reliability of test results. According to the results of the external quality evaluation conducted by the Clinical Laboratory Center of the National Health Commission on March 16-24, 2020, of the 844 laboratories that received valid results, 701 (83.1%) were qualified, and 143 (16.9%) were not. Qualified, the overall laboratory testing conditions are good, but different laboratories still have differences in personnel operation ability, single-target positive sample interpretation ability, and quality control.
How to reduce the false negative of SARS-CoV-2 nucleic acid detection?
Reducing false negatives in nucleic acid detection should be optimized from the four aspects of producing false negatives.
(1)There is a certain amount of virus in the cells of the infected person. The concentration of the virus in different parts of the body of suspected infected persons will be different at different times. If there is no pharynx, it may be in bronchial lavage fluid or feces. If multiple types of samples can be collected at the same time or at different stages of disease progression for testing, Will help avoid false negatives.
(2) Cells containing virus should be collected during sample collection. This problem can be solved to a large extent by strengthening the training of sample collectors.
(3) Reliable IVD reagents. By carrying out research on the detection performance evaluation of reagents at the national level, and discussing the existing problems, the detection efficiency of reagents can be further improved and the sensitivity of analysis can be improved.
(4) Standardized operation of clinical laboratories. By strengthening the training of laboratory personnel, continuously improving the laboratory quality management system, ensuring reasonable divisions, and improving the ability of personnel to detect, it is possible to reduce false negatives due to improper laboratory operations.
Reasons for the re-test positive of SARS-CoV-2 nucleic acid test in recovered and discharged patients
The “COVID-19 Diagnosis and Treatment Plan (Trial Seventh Edition)” clearly stipulates that one of the criteria for COVID-19 patients to be cured and discharged from the hospital is that two consecutive respiratory tract samples have a negative nucleic acid test (at least 24 hours apart), but there are very few The SARS-CoV-2 nucleic acid test was positive again in discharged patients due to various reasons.
(1)SARS-CoV-2 is a new virus. It is necessary to further understand its pathogenic mechanism, the full picture of the disease caused and the characteristics of the disease course. Therefore, on the one hand, it is necessary to strengthen the management of discharged patients and conduct 14-day medical observation. Carry out follow-up, health monitoring and health guidance to deepen the understanding of the whole process of the occurrence, development and outcome of the disease.
(2)The patient may be infected with the virus again. Academician Zhong Nanshan said: Because cured patients have antibodies, SARS-CoV-2 can be eliminated by antibodies when they invade again. There are many reasons, which may be the cause of the recovered patient, or it may be related to the mutation of the virus, or even the cause of laboratory testing. If it is the virus itself, the SARS-CoV-2 mutation may cause the antibody produced by the recovered patient to be ineffective against the mutated virus. If the patient is infected with the mutated virus again, the nucleic acid test may be positive again.
(3)As far as laboratory testing methods are concerned, each testing method has its limitations. SARS-CoV-2 nucleic acid detection is due to the choice of gene sequence, the composition of reagents, the sensitivity of the method and other reasons, leading to the existing kits have their own lower detection limits. After the patient is treated, the virus in the body decreases. When the viral load in the sample to be tested is below the lower limit of detection, a “negative” result will appear. However, this result does not mean that the virus in the body has completely disappeared. The virus may be after the treatment is stopped. Resurgence”, continue to copy. Therefore, it is recommended to review once a week within 2 to 4 weeks after discharge.
(4) Nucleic acid is the genetic material of the virus. The virus is killed after the patient has undergone antiviral treatment, but the remaining viral RNA fragments are still retained in the human body, and are not completely discharged from the body. Sometimes, under certain circumstances, it can be more retained. A long time, and at this time the nucleic acid test will be “transient” positive. With the extension of the patient’s recovery time, after the residual RNA fragments in the body are gradually exhausted, the nucleic acid test result can turn negative.
(5) The nucleic acid test result of SARS-CoV-2 only proves the presence or absence of viral RNA, and cannot prove the activity of the virus and whether the virus is transmissible. It is necessary to prove whether a patient who has a positive nucleic acid test again will become a source of infection again. It is necessary to carry out virus culture on clinical samples and cultivate a “live” virus to prove that it is infectious.
Summary
In summary, SARS-CoV-2 nucleic acid test false negatives, retest positives, and other conditions that are inconsistent with clinical manifestations cannot be completely avoided. In actual screening and testing, it is recommended to combine clinical symptoms, imaging examinations (CT) and experiments Laboratory test (nucleic acid test + virus-specific antibody test) results for comprehensive diagnosis to prevent missed diagnosis and misdiagnosis. If the test results are found to be obviously inconsistent with the clinical manifestations, it is recommended to conduct a comprehensive analysis of the entire test link (sample collection, circulation and processing links) to exclude SARS-CoV-2 virus early infection, recurrent infection or combined with other respiratory virus infections, etc. possible. If conditions permit, it is recommended to collect more sensitive samples such as sputum or alveolar lavage fluid for re-examination.
Related Products:
SARS-CoV-2 Nucleic Acid Detection Kit (Multiplex PCR Fluorescent Probe Method)
Post time: Sep-03-2021