Genes are the basic genetic units that control traits. Except for the genes of some viruses, which are composed of RNA, the genes of most organisms are composed of DNA . Most diseases of organisms are caused by the interaction between genes and the environment. Gene therapy can essentially cure or alleviate many diseases. Gene therapy is considered to be a revolution in the field of medicine and pharmacy . Gene therapy drugs in a broad sense include based on DNA-modified DNA drugs (such as in vivo gene therapy drugs based on viral vectors, in vitro gene therapy drugs, naked plasmid drugs, etc.) and RNA drugs (such as antisense oligonucleotide drugs, siRNA drugs, and mRNA gene therapy, etc.); narrow sense Gene therapy drugs mainly include plasmid DNA drugs, gene therapy drugs based on viral vectors, gene therapy drugs based on bacterial vectors, gene editing systems, and cell therapy drugs for in vitro gene modification. After years of tortuous development, gene therapy drugs have achieved encouraging clinical results. (Not counting DNA vaccines and mRNA vaccines) At present, 45 gene therapy drugs have been approved for marketing in the world. A total of 9 gene therapies have been approved for marketing this year, including 7 gene therapies approved for marketing for the first time this year, namely: CARVYKTI, Amvuttra, Upstaza, Roctavian, Hemgenix, Adstiladrin and Ebvallo, (Note: The other two were approved in the United States this year. The first batch of gene therapies to be marketed are: ① Zynteglo, which was approved by the FDA for marketing in the United States in August 2022, and was approved for marketing by the European Union in 2019; .) With the launch of more and more gene therapy products and the rapid development of gene therapy technology, gene therapy is about to usher in a period of rapid development.
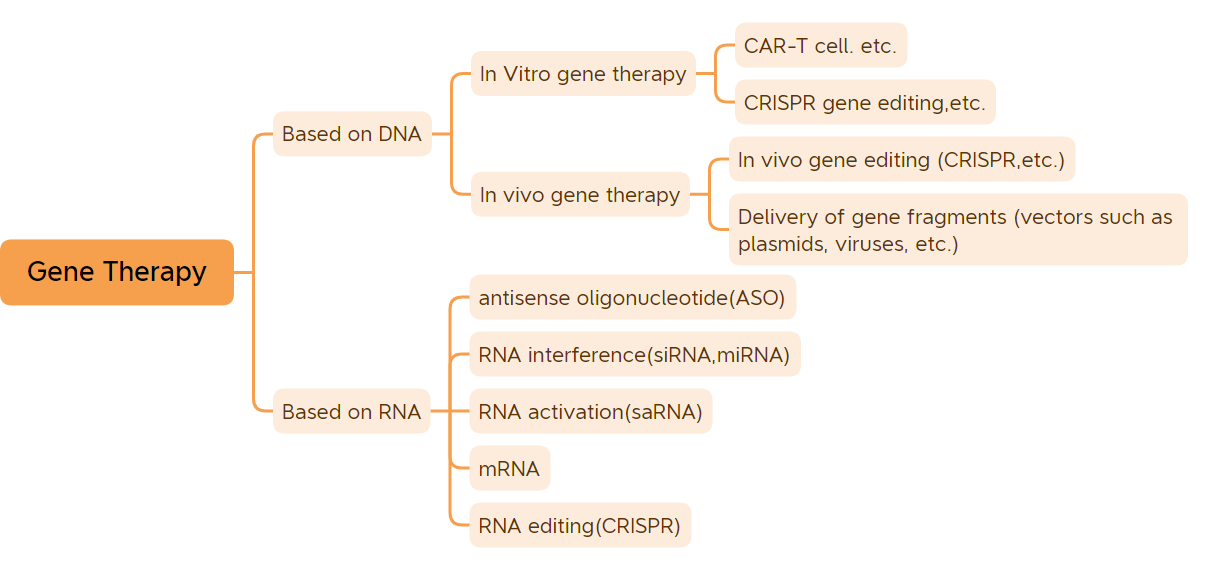
Classification of gene therapy (Image source: Bio-Matrix)
This article lists 45 gene therapies (excluding DNA vaccines and mRNA vaccines) that have been approved for marketing.
1. In vitro gene therapy
(1) Strimvelis
Company: Developed by GlaxoSmithKline (GSK).
Time to market: It was approved for marketing by the European Union in May 2016.
Indications: For the treatment of severe combined immunodeficiency (SCID).
Remarks: The general process of this therapy is to first obtain the patient’s own hematopoietic stem cells, expand and culture them in vitro, then use retrovirus to introduce a functional ADA (adenosine deaminase) gene copy into the hematopoietic stem cells, and finally inject the modified Hematopoietic stem cells are reinfused back into the body. Clinical results show that the 3-year survival rate of ADA-SCID patients treated with Strimvelis is 100%.
(2) Zalmoxis
Company: Produced by Italy MolMed Company.
Time to market: Obtained conditional marketing authorization from the European Union in 2016.
Indications: It is used for adjuvant therapy of the immune system of patients after hematopoietic stem cell transplantation.
Remarks: Zalmoxis is an allogeneic T cell suicide gene immunotherapy modified by retroviral vectors. This method uses retroviral vectors to genetically modify allogeneic T cells, so that the genetically modified T cells express 1NGFR and HSV-TK Mut2 suicide genes allow people to use ganciclovir (ganciclovir) drugs at any time to kill T cells that cause adverse immune reactions, prevent possible further deterioration of GVHD, and provide postoperative immune function reconstruction for haploidentical HSCT patients Escort.
(3) Invossa-K
Company: Developed by TissueGene (KolonTissueGene).
Time to market: Approved for listing in South Korea in July 2017.
Indications: For the treatment of degenerative knee arthritis.
Remarks: Invossa-K is an allogeneic cell gene therapy involving human chondrocytes. The allogeneic cells are genetically modified in vitro, and the modified cells can express and secrete transforming growth factor β1 (TGF-β1) after intra-articular injection. β1), thereby improving the symptoms of osteoarthritis. Clinical results show that Invossa-K can significantly improve knee arthritis. It was revoked in 2019 by the Korean Food and Drug Administration because the manufacturer mislabeled the ingredients used.
(4) Zynteglo
Company: Researched and developed by bluebird bio.
Time to market: Approved by the European Union for marketing in 2019, and approved by the FDA for marketing in the United States in August 2022.
Indications: For the treatment of transfusion-dependent β-thalassemia.
Remarks: Zynteglo is a lentiviral in vitro gene therapy that introduces a functional copy of the normal β-globin gene (βA-T87Q-globin gene) into hematopoietic stem cells taken from the patient through a lentiviral vector , and then reinfuse these genetically modified autologous hematopoietic stem cells into the patient. Once the patient has a normal βA-T87Q-globin gene, they may produce normal HbAT87Q protein, which can effectively reduce or eliminate the need for blood transfusion. It’s a one-time therapy designed to replace lifelong blood transfusions and lifelong medications for patients 12 years of age and older.
(5) Skysona
Company: Researched and developed by bluebird bio.
Time to market: Approved by the European Union for marketing in July 2021, and approved by the FDA for marketing in the United States in September 2022.
Indications: For the treatment of early cerebral adrenoleukodystrophy (CALD).
Remarks: Skysona gene therapy is the only one-time gene therapy approved for the treatment of early-stage cerebral adrenoleukodystrophy (CALD). Skysona (elivaldogene autotemcel, Lenti-D) is a hematopoietic stem cell lentiviral in vitro gene therapy Lenti-D. The general procedure of the therapy is as follows: autologous hematopoietic stem cells are taken out from the patient, transduced and modified by lentivirus carrying the human ABCD1 gene in vitro, and then reinfused back to the patient. It is used to treat patients under the age of 18, carrying ABCD1 gene mutations, and CALD.
(6) Kymriah
COMPANY: Developed by Novartis.
Time to market: Approved by the FDA for marketing in August 2017.
Indications: Treatment of precursor B-cell acute lymphoblastic leukemia (ALL) and relapsed and refractory DLBCL.
Remarks: Kymriah is a lentiviral in vitro gene therapy drug, the first CAR-T therapy approved for marketing in the world, targeting CD19, and using 4-1BB co-stimulatory factor. It’s priced at $475,000 in the U.S. and $313,000 in Japan.
(7) Yescarta
Company: Developed by Kite Pharma, a subsidiary of Gilead (GILD).
Time to market: Approved by the FDA for marketing in October 2017; Fosun Kite introduced Yescarta technology from Kite Pharma and produced it in China after obtaining authorization. Approved for listing in the country.
Indications: For the treatment of relapsed or refractory large B-cell lymphoma.
Remarks: Yescarta is a retroviral in vitro gene therapy, which is the second approved CAR-T therapy in the world. It targets CD19 and adopts the costimulator of CD28. It’s priced at $373,000 in the United States.
(8) Tecartus
Company: Developed by Gilead (GILD).
Time to market: Approved by the FDA for marketing in July 2020.
Indications: For relapsed or refractory mantle cell lymphoma.
Remarks: Tecartus is an autologous CAR-T cell therapy targeting CD19, and it is the third CAR-T therapy approved for marketing in the world.
(9) Breyanzi
COMPANY: Developed by Bristol-Myers Squibb (BMS).
Time to market: Approved by the FDA for marketing in February 2021.
Indications: Relapsed or refractory (R/R) large B-cell lymphoma (LBCL).
Remarks: Breyanzi is an in vitro gene therapy based on lentivirus, the fourth CAR-T therapy approved for marketing in the world, targeting CD19. The approval of Breyanzi is a milestone for Bristol-Myers Squibb in the field of cellular immunotherapy, which it acquired when it acquired Celgene for $74 billion in 2019.
(10) Abecma
Company: Co-developed by Bristol-Myers Squibb (BMS) and bluebird bio.
Time to market: Approved by the FDA for marketing in March 2021.
Indications: relapsed or refractory multiple myeloma.
Remarks: Abecma is an in vitro gene therapy based on lentivirus, the world’s first CAR-T cell therapy targeting BCMA, and the fifth CAR-T therapy approved by FDA. The principle of the drug is to express chimeric BCMA receptors on the patient’s own T cells through lentivirus-mediated gene modification in vitro. Treatment to eliminate non-genetically modified T cells in patients, and then reinfuse modified T cells, which seek and kill BCMA-expressing cancer cells in patients.
(11) Libmeldy
COMPANY: Developed by Orchard Therapeutics.
Time to market: Approved by the European Union for listing in December 2020.
Indications: For the treatment of metachromatic leukodystrophy (MLD).
Remarks: Libmeldy is a gene therapy based on autologous CD34+ cells genetically modified in vitro by lentivirus. Clinical data show that a single intravenous infusion of Libmeldy can effectively alter the course of early-onset MLD compared with severe motor and cognitive impairment in untreated patients of the same age.
(12) Benoda
Company: Developed by WuXi Giant Nuo.
Time to market: Officially approved by NMPA in September 2021.
Indications: Treatment of adult patients with relapsed or refractory large B-cell lymphoma (r/r LBCL) after second-line or above systemic therapy.
Remarks: Beinoda is an anti-CD19 CAR-T gene therapy, and it is also the core product of WuXi Juro Company. It is the second CAR-T product approved in China, except for relapsed/refractory large B-cell lymphoid WuXi Giant Nuo also plans to develop Regiorensai injection for the treatment of multiple other indications, including follicular lymphoma (FL), mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL) , second-line diffuse large B-cell lymphoma (DLBCL) and acute lymphoblastic leukemia (ALL).
(13) CARVYKTI
Company: Legend Biotech’s first product approved for marketing.
Time to market: Approved by the FDA for marketing in February 2022.
Indications: for the treatment of relapsed or refractory multiple myeloma (R/R MM).
Remarks: CARVYKTI (ciltacabtagene autoleucel, Cilta-cel for short) is a CAR-T cell immune gene therapy with two single-domain antibodies targeting B-cell maturation antigen (BCMA). Data show that CARVYKTI In patients with relapsed or refractory multiple myeloma who have received four or more prior therapies (including proteasome inhibitors, immunomodulators and anti-CD38 monoclonal antibodies), an overall response rate of 98% has been shown.
(14) Ebvallo
COMPANY: Developed by Atara Biotherapeutics.
the European Commission (EC) for marketing in December 2022 , it is the world’s first universal T cell therapy approved for marketing.
Indications: As a monotherapy for Epstein-Barr virus (EBV)-related post-transplantation lymphoproliferative disease (EBV+PTLD), the patients receiving treatment must be adults and children over 2 years old who have previously received at least one other drug therapy.
Remarks: Ebvallo is an allogeneic EBV-specific universal T-cell gene therapy that targets and eliminates EBV-infected cells in an HLA-restricted manner. The approval of this therapy is based on the results of the pivotal phase 3 clinical trial study, and the results showed that the ORR of the HCT group and the SOT group was 50%. The complete remission (CR) rate was 26.3%, the partial remission (PR) rate was 23.7%, and the median time to remission (TTR) was 1.1 months. Of the 19 patients who achieved remission, 11 had a duration of response (DOR) of more than 6 months. In addition, in terms of safety, no adverse reactions such as graft-versus-host disease (GvHD) or Ebvallo-related cytokine release syndrome occurred.
2. In vivo gene therapy based on viral vectors
(1) Gendicine/Jin Sheng
Company: Developed by Shenzhen Saibainuo Company.
Time to market: Approved to be listed in China in 2003.
Indications: For the treatment of head and neck squamous cell carcinoma.
Note: Recombinant human p53 adenovirus injection Gendicine/Jinyousheng is an adenovirus vector gene therapy drug with independent intellectual property rights owned by Shenzhen Saibainuo Company. Human type 5 adenovirus is composed of human adenovirus type 5. The former is the main structure for the anti-tumor effect of the drug, and the latter mainly acts as a carrier. The adenovirus vector carries the therapeutic gene p53 into the target cell, expresses the tumor suppressor gene p53 in the target cell, and its gene expression The product can up-regulate a variety of anti-cancer genes and down-regulate the activities of a variety of oncogenes, thereby enhancing the body’s tumor suppressor effect and achieving the purpose of killing tumors.
(2) Rigvir
Company: Developed by Latima Company, Latvia.
Listing time: Approved for listing in Latvia in 2004.
Indications: For the treatment of melanoma.
Remarks: Rigvir is a gene therapy based on a genetically modified ECHO-7 enterovirus vector. At present, the drug has been adopted in Latvia, Estonia, Poland, Armenia, Belarus, etc., and is also undergoing EMA registration in EU countries . Clinical cases in the past ten years have proved that Rigvir oncolytic virus is safe and effective, and can increase the survival rate of melanoma patients by 4-6 times. In addition, this therapy is also applicable to a variety of other cancers, including colorectal cancer, pancreatic cancer, bladder cancer Cancer, kidney cancer, prostate cancer, lung cancer, uterine cancer, lymphosarcoma, etc.
(3) Oncorine
Company: Developed by Shanghai Sanwei Biological Company.
Time to market: Approved to be listed in China in 2005.
Indications: treatment of head and neck tumors, liver cancer, pancreatic cancer, cervical cancer and other cancers.
Remarks: Oncorine (安科瑞) is an oncolytic virus gene therapy product using adenovirus as a carrier. An oncolytic adenovirus is obtained, which can specifically replicate in p53 gene deficient or abnormal tumors, leading to the lysis of tumor cells, thereby killing tumor cells. without damaging normal cells. Clinical studies have shown that Ankerui has good safety and efficacy for a variety of malignant tumors.
(4) Glybera
Company: Developed by uniQure.
Time to market: Approved for listing in Europe in 2012.
Indications: Treatment of lipoprotein lipase deficiency (LPLD) with severe or recurrent episodes of pancreatitis despite a strictly restricted fat diet.
Remarks: Glybera (alipogene tiparvovec) is a gene therapy drug based on AAV, which uses AAV as a carrier to transduce the therapeutic gene LPL into muscle cells, so that the corresponding cells can produce a certain amount of lipoprotein lipase, To alleviate the disease, this therapy is effective for a long time (drug effect can last for many years) once administered. The drug was withdrawn from the market in 2017. The reason for its withdrawal may be related to two factors: high pricing and limited market demand. The average treatment cost of the drug is as high as US$1 million, and only one patient has purchased and used it so far. Although the medical insurance company has reimbursed US$900,000 for it, it is also a relatively large burden for the insurance company. In addition, the indications targeted by the drug are too rare, with an incidence rate of about 1 in 1 million and a high rate of misdiagnosis.
(5) Imlygic
Company: Developed by Amgen.
Time to market: In 2015, it was approved to be listed in the United States and the European Union.
Indications: Treatment of melanoma lesions that cannot be completely removed by surgery.
Remarks: Imlygic is an attenuated herpes simplex virus type 1 that has been modified by genetic technology (deleting its ICP34.5 and ICP47 gene fragments, and inserting the human granulocyte macrophage colony-stimulating factor GM-CSF gene into the virus) (HSV-1) oncolytic virus is the first FDA-approved oncolytic virus gene therapy. The method of administration is intralesional injection, which can be directly injected into melanoma lesions to cause the rupture of tumor cells, release tumor-derived antigens and GM-CSF, and promote anti-tumor immune responses.
(6) Luxturna
Company: Developed by Spark Therapeutics, a subsidiary of Roche.
Time to market: It was approved for marketing by the FDA in 2017, and then approved for marketing in Europe in 2018.
Indications: For the treatment of children and adults who have lost vision due to double-copy RPE65 gene mutations but retain a sufficient number of viable retinal cells.
Remarks: Luxturna is an AAV-based gene therapy administered by subretinal injection. The gene therapy uses AAV2 as a carrier to introduce a functional copy of the normal RPE65 gene into the patient’s retinal cells, so that the corresponding cells express the normal RPE65 protein, making up for the patient’s RPE65 protein deficiency, thereby improving the patient’s vision.
(7) Zolgensma
Company: Developed by AveXis, a subsidiary of Novartis.
Time to market: Approved by the FDA for marketing in May 2019.
Indications: Treatment of Spinal Muscular Atrophy (SMA) patients under 2 years old.
Remarks: Zolgensma is a gene therapy based on AAV vector. This drug is the only one-time treatment plan for spinal muscular atrophy approved for marketing in the world. The launch of the drug opens a new era in the treatment of spinal muscular atrophy. page, is a milestone progress. This gene therapy uses the scAAV9 vector to introduce the normal SMN1 gene into the patient through intravenous infusion to produce normal SMN1 protein, thereby improving the function of affected cells such as motor neurons. In contrast, the SMA drugs Spinraza and Evrysdi require long-term repeated dosing. Spinraza is given by spinal injection every four months, and Evrysdi is a daily oral drug.
(8) Delytact
Company: Developed by Daiichi Sankyo Company Limited (TYO: 4568).
Time to market: Conditional approval from Japan’s Ministry of Health, Labor and Welfare (MHLW) in June 2021.
Indications: For the treatment of malignant glioma.
Remarks: Delytact is the fourth oncolytic virus gene therapy product approved globally, and the first oncolytic virus product approved for the treatment of malignant glioma. Delytact is a genetically engineered herpes simplex virus type 1 (HSV-1) oncolytic virus developed by Dr. Todo and colleagues. Delytact introduces additional deletion mutations into the G207 genome of the second-generation HSV-1, enhancing its selective replication in cancer cells and the induction of anti-tumor immune responses while maintaining high safety. Delytact is the first third-generation oncolytic HSV-1 currently undergoing clinical evaluation. The approval of Delytact in Japan is mainly based on a single-arm phase 2 clinical trial. In patients with recurrent glioblastoma, Delytact achieved the primary endpoint of one-year survival rate, and the results showed that Delytact showed better efficacy compared with G207. Strong replicative force and higher antitumor activity. This was effective in solid tumor models of breast, prostate, schwannomas, nasopharyngeal, hepatocellular, colorectal, malignant peripheral nerve sheath tumors, and thyroid cancer.
(9) Upstaza
COMPANY: Developed by PTC Therapeutics, Inc. (NASDAQ: PTCT).
Time to market: Approved by the European Union for marketing in July 2022.
Indications: For aromatic L-amino acid decarboxylase (AADC) deficiency, it is approved for the treatment of patients aged 18 months and older.
Remarks: Upstaza™ (eladocagene exuparvovec) is an in vivo gene therapy with adeno-associated virus type 2 (AAV2) as the carrier. Patients become sick due to mutations in the gene encoding the AADC enzyme. AAV2 carries a healthy gene encoding the AADC enzyme. The form of gene compensation achieves a therapeutic effect. In theory, one administration is effective for a long time. It is the first marketed gene therapy that is directly injected into the brain. The marketing authorization is applicable to all 27 EU member states, as well as Iceland, Norway and Liechtenstein.
(10) Roctavian
Company: Developed by BioMarin Pharmaceutical (BioMarin).
Time to market: Approved for marketing by the European Union in August 2022; marketing authorization by the UK Medicines and Healthcare products Administration (MHRA) in November 2022.
Indications: For the treatment of adult patients with severe hemophilia A who have no history of FVIII factor inhibition and are negative for AAV5 antibodies.
Remarks: Roctavian (valoctocogene roxaparvovec) uses AAV5 as a vector and uses the human liver-specific promoter HLP to drive the expression of human coagulation factor VIII (FVIII) with B domain deleted. The European Commission’s decision to approve the marketing of valoctocogene roxaparvovec is based on the overall data of the drug’s clinical development project. Among them, the results of the phase III clinical trial GENER8-1 showed that compared with the data of the year before enrollment, after a single infusion of valoctocogene roxaparvovec, The subject’s annual bleeding rate (ABR) is significantly reduced, the frequency of use of recombinant coagulation factor VIII (F8) protein preparations is reduced, or the activity of F8 in the blood in the body is significantly increased. After 4 weeks of treatment, the subject’s annual F8 usage rate and ABR requiring treatment were reduced by 99% and 84%, respectively, and the difference was statistically significant (p<0.001). The safety profile was good, and no subject experienced F8 factor inhibition, malignancy or thrombosis side effects, and no treatment-related serious adverse events (SAEs) were reported.
(11) Hemgenix
Company: Developed by UniQure Corporation.
Time to market: Approved by the FDA for marketing in November 2022.
Indications: For the treatment of adult patients with hemophilia B.
Remarks: Hemgenix is a gene therapy based on the AAV5 vector. The drug is equipped with the coagulation factor IX (FIX) gene variant FIX-Padua, which is administered intravenously. After administration, the gene can express the FIX coagulation factor in the liver and secrete After entering the blood to exert the coagulation function, so as to achieve the purpose of treatment, theoretically, one administration is effective for a long time.
(12) Adstiladrin
Company: Developed by Ferring Pharmaceuticals .
Time to market: Approved by the FDA for marketing in December 2022.
Indications: For the treatment of high-risk non-muscle-invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG) .
Remarks: Adstiladrin is a gene therapy based on a non-replicating adenoviral vector, which can overexpress interferon alfa-2b protein in target cells, and is administered through a urinary catheter into the bladder (administered once every three months) , the virus vector can effectively infect into the cells of the bladder wall, and then overexpress the interferon alfa-2b protein to exert a therapeutic effect. This novel gene therapy method thus transforms the patient’s own bladder wall cells into a miniature “factory” that produces interferon, thereby enhancing the patient’s ability to fight cancer.
The safety and efficacy of Adstiladrin were evaluated in a multicenter clinical study including 157 patients with high-risk BCG-nonresponsive NMIBC. Patients received Adstiladrin every three months for up to 12 months, or until unacceptable toxicity to treatment or recurrence of high-grade NMIBC. Overall, 51 percent of enrolled patients treated with Adstiladrin achieved a complete response (disappearance of all signs of cancer seen on cystoscopy, biopsy tissue, and urine).
3. Small nucleic acid drugs
(1) Vitravene
Company: Jointly developed by Ionis Pharma (formerly Isis Pharma) and Novartis.
Time to market: In 1998 and 1999, it was approved for marketing by FDA and EU EMA.
Indications: For the treatment of cytomegalovirus retinitis in HIV-positive patients.
Remarks: Vitravene is an antisense oligonucleotide drug, which is the first oligonucleotide drug approved for marketing in the world. At the initial stage of listing, the market demand for anti-CMV drugs was very urgent; later, due to the development of highly active antiretroviral therapy, the number of CMV cases dropped sharply. Due to the sluggish market demand, the drug was launched in 2002 and 2006 Withdrawal in EU countries and the United States.
(2) Macugen
Company: Co-developed by Pfizer and Eyetech.
Time to market: Approved for listing in the United States in 2004.
Indications: For the treatment of neovascular age-related macular degeneration.
Remarks: Macugen is a pegylated modified oligonucleotide drug, which can target and bind vascular endothelial growth factor (VEGF165 subtype), and the administration method is intravitreal injection.
(3) Defitelio
Company: Developed by Jazz Pharmaceuticals.
Time to market: It was approved for marketing by the European Union in 2013 and approved by the FDA for marketing in March 2016.
Indications: For the treatment of hepatic veno-occlusive disease associated with renal or pulmonary dysfunction after hematopoietic stem cell transplantation.
Remarks: Defitelio is an oligonucleotide drug, which is a mixture of oligonucleotides with plasmin properties. Withdrawn from the market in 2009 for commercial reasons.
(4) Kynamro
Company: Co-developed by Ionis Pharma and Kastle.
Time to market: In 2013, it was approved for marketing in the United States as an orphan drug.
Indications: For the adjuvant treatment of homozygous familial hypercholesterolemia.
Remarks: Kynamro is an antisense oligonucleotide drug, which is an antisense oligonucleotide targeting human apo B-100 mRNA. Kynamro is administered as 200 mg subcutaneously once a week.
(5) Spinraza
Company: Developed by Ionis Pharmaceuticals.
Time to market: Approved by the FDA for marketing in December 2016.
Indications: For the treatment of spinal muscular atrophy (SMA).
Remarks: Spinraza (nusinersen) is an antisense oligonucleotide drug. By binding to the cleavage site of SMN2 exon 7, Spinraza can change the RNA cleavage of SMN2 gene, thereby increasing the production of fully functional SMN protein . In August 2016, BIOGEN exercised its option to acquire the global rights to Spinraza. Spinraza only started its first clinical trial in humans in 2011. In just 5 years, it was approved by the FDA for marketing in 2016, which reflects the FDA’s full recognition of its efficacy. The drug was approved for marketing in China in April 2019. The entire approval cycle for Spinraza in China was less than 6 months, and it was 2 years and 2 months since Spinraza was first approved in the United States. The speed of listing in China is already very fast. This is also due to the fact that the Center for Drug Evaluation issued the “Notice on Publishing the List of the First Batch of Overseas New Drugs Urgently Needed in Clinical Practice” on November 1, 2018, and was included in the first batch of 40 foreign new drugs for accelerated review, among which Spinraza was ranked in.
(6) Exondys 51
Company: Developed by AVI BioPharma (later renamed Sarepta Therapeutics).
Time to market: In September 2016, it was approved for marketing by the FDA.
Indications: For the treatment of Duchenne muscular dystrophy (DMD) with exon 51 skipping gene mutation in the DMD gene.
Remarks: Exondys 51 is an antisense oligonucleotide drug, the antisense oligonucleotide can bind to the position of exon 51 of pre-mRNA of DMD gene, resulting in the formation of mature mRNA, part of exon 51 is blocked Excision, thereby partially correcting the mRNA reading frame, helping patients to synthesize some functional forms of dystrophin that is shorter than the normal protein, thereby improving the patient’s symptoms.
(7) Tegsedi
Company: Developed by Ionis Pharmaceuticals.
Time to market: It was approved for marketing by the European Union in July 2018.
Indications: For the treatment of hereditary transthyretin amyloidosis (hATTR).
Remarks: Tegsedi is an antisense oligonucleotide drug targeting transthyretin mRNA. It is the first drug approved in the world for the treatment of hATTR. It is administered by subcutaneous injection. The drug reduces the production of ATTR protein by targeting the mRNA of transthyretin (ATTR), and has a good benefit-risk ratio in the treatment of ATTR, and the patient’s neuropathy and quality of life have been substantially improved, and it is compatible with TTR mutation types, Neither disease stage nor presence of cardiomyopathy was relevant.
(8) Onpattro
Company: Jointly developed by Alnylam Corporation and Sanofi Corporation.
Time to market: Approved for listing in the United States in 2018.
Indications: For the treatment of hereditary transthyretin amyloidosis (hATTR).
Remarks: Onpattro is an siRNA drug targeting transthyretin mRNA, which reduces the production of ATTR protein in the liver and reduces the accumulation of amyloid deposits in peripheral nerves by targeting the mRNA of transthyretin (ATTR) , thereby improving and alleviating disease symptoms.
(9) Givlaari
Company: Developed by Alnylam Corporation.
Time to market: Approved by the FDA for marketing in November 2019.
Indications: For the treatment of acute hepatic porphyria (AHP) in adults.
Remarks: Givlaari is an siRNA drug, which is the second siRNA drug approved for marketing after Onpattro. The way of administration is subcutaneous injection. The drug targets the mRNA of ALAS1 protein, and monthly treatment with Givlaari can significantly and permanently reduce the level of ALAS1 in the liver, thereby reducing the levels of neurotoxic ALA and PBG to the normal range, thereby Alleviate the symptoms of the patient’s disease. The data showed that patients treated with Givlaari had a 74% reduction in the number of seizures compared to the placebo group.
(10) Vyondys53
COMPANY: Developed by Sarepta Therapeutics.
Time to market: Approved by the FDA for marketing in December 2019.
Indications: For the treatment of DMD patients with dystrophin gene exon 53 splicing mutation.
Remarks: Vyondys 53 is an antisense oligonucleotide drug, which targets the splicing process of dystrophin pre-mRNA. Exon 53 is partially truncated, i.e. not present on the mature mRNA, and is designed to produce a truncated but still functional dystrophin, thereby improving exercise capacity in patients.
(11) Waylivra
Company: Developed by Ionis Pharmaceuticals and its subsidiary Akcea Therapeutics.
Time to market: It was approved for marketing by the European Medicines Agency (EMA) in May 2019.
Indications: As an adjuvant therapy in addition to diet control in adult patients with familial chylomicronemia syndrome (FCS).
Remarks: Waylivra is an antisense oligonucleotide drug, which is the first drug approved for marketing in the world for the treatment of FCS.
(12) Leqvio
Company: Developed by Novartis.
Time to market: Approved by the European Union for marketing in December 2020.
Indications: For the treatment of adults with primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia.
Remarks: Leqvio is an siRNA drug targeting PCSK9 mRNA. It is the world’s first siRNA therapy for lowering cholesterol (LDL-C). It is administered by subcutaneous injection. The drug reduces the level of PCSK9 protein through RNA interference, thereby reducing the level of LDL-C. Clinical data show that for patients who cannot reduce LDL-C levels to the target level after treatment with the maximum tolerated dose of statins, Leqvio can reduce LDL-C by about 50%.
(13)Oxlumo
Company: Developed by Alnylam Pharmaceuticals.
Time to market: Approved by the European Union for marketing in November 2020.
Indications: For the treatment of primary hyperoxaluria type 1 (PH1).
Remarks: Oxlumo is an siRNA drug targeting hydroxyacid oxidase 1 (HAO1) mRNA, and the administration method is subcutaneous injection. The drug was developed using Alnylam’s latest enhanced stabilization chemistry, ESC-GalNAc conjugation technology, which enables subcutaneously administered siRNA with greater persistence and potency. The drug degrades or inhibits hydroxyacid oxidase 1 (HAO1) mRNA, reduces the level of glycolate oxidase in the liver, and then consumes the substrate required for the production of oxalate, reducing oxalate production to control the progression of the disease in patients and improve disease symptoms.
(14) Viltepso
Company: Developed by NS Pharma, a subsidiary of Nippon Shinyaku.
Time to market: Approved by the FDA for marketing in August 2020.
Indications: For the treatment of Duchenne muscular dystrophy (DMD) with exon 53 skipping gene mutation in the DMD gene.
Remarks: Viltepso is an antisense oligonucleotide drug that can bind to the position of exon 53 of the pre-mRNA of the DMD gene, causing part of exon 53 to be excised after the formation of mature mRNA, thereby partially correcting the mRNA reading frame The box helps patients to synthesize some functional forms of dystrophin that are shorter than normal proteins, thereby improving the symptoms of patients.
(15) Amondys 45
Company: Developed by Sarepta Therapeutics.
Time to market: Approved by the FDA for marketing in February 2021.
Indications: For the treatment of Duchenne muscular dystrophy (DMD) with exon 45 skipping gene mutation in the DMD gene.
Remarks: Amondys 45 is an antisense oligonucleotide drug, the antisense oligonucleotide can bind to the position of exon 45 of the pre-mRNA of DMD gene, resulting in the part of exon 45 being blocked after the formation of mature mRNA Excision, thereby partially correcting the mRNA reading frame, helping patients to synthesize some functional forms of dystrophin that is shorter than the normal protein, thereby improving the patient’s symptoms.
(16) Amvuttra (vutrisiran)
Company: Developed by Alnylam Pharmaceuticals.
Time to market: Approved by the FDA for marketing in June 2022.
Indications: For the treatment of hereditary transthyretin amyloidosis with polyneuropathy (hATTR-PN) in adults.
Remarks: Amvuttra (Vutrisiran) is an siRNA drug targeting transthyretin (ATTR) mRNA, administered by subcutaneous injection. Vutrisiran is based on Alnylam’s Enhanced Stability Chemistry (ESC)-GalNAc conjugate delivery platform design with increased potency and metabolic stability. The approval of the therapy is based on the 9-month data of its Phase III clinical study (HELIOS-A), and the overall results show that the therapy improved the symptoms of hATTR-PN, and more than 50% of the patients’ condition was reversed or stopped from worsening.
4. Other gene therapy drugs
(1) Rexin-G
Company: Developed by Epeius Biotech.
Time to market: In 2005, it was approved for marketing by the Philippine Food and Drug Administration (BFAD).
Indications: For the treatment of advanced cancers resistant to chemotherapy.
Remarks: Rexin-G is a gene-loaded nanoparticle injection. It introduces the cyclin G1 mutant gene into the target cells through a retroviral vector to specifically kill solid tumors. The administration method is intravenous infusion. As a tumor-targeted drug that actively seeks out and destroys metastatic cancer cells, it has a certain curative effect on patients who have failed other cancer drugs, including targeted biologics.
(2) Neovasculgen
Company: Developed by Human stem cells institute.
Listing time: It was approved for listing in Russia on December 7, 2011, and then launched in Ukraine in 2013.
Indications: For the treatment of peripheral vascular arterial disease, including severe limb ischemia.
Remarks: Neovasculgen is a gene therapy based on DNA plasmids. The vascular endothelial growth factor (VEGF) 165 gene is constructed on the plasmid backbone and infused into patients.
(3) Collategene
Company: Jointly developed by Osaka University and venture capital companies.
Time to market: Approved by the Ministry of Health, Labor and Welfare of Japan in August 2019.
Indications: Treatment of critical lower extremity ischemia.
Remarks: Collategene is a plasmid-based gene therapy, the first domestic gene therapy drug produced by AnGes, a gene therapy company in Japan. The main component of this drug is a naked plasmid containing the human hepatocyte growth factor (HGF) gene sequence. If the drug is injected into the muscles of the lower limbs, the expressed HGF will promote the formation of new blood vessels around the occluded blood vessels. Clinical trials have confirmed its effect on improving ulcers.
How Foregene can help the gene therapy development?
We help saving the screening time in large scale screening, at the early stage of siRNA drug development.
More details visit:
https://www.foreivd.com/cell-direct-rt-qpcr-kit-direct-rt-qpcr-series/
Post time: Dec-27-2022