Antibodies, also called immunoglobulins (Ig), are glycoproteins that specifically bind to antigens.
Conventional antibody preparation is produced by immunizing animals and collecting antiserum. Therefore, antiserum usually contains antibodies against other unrelated antigens and other protein components in the serum. General antigen molecules mostly contain multiple different epitopes, so conventional antibodies are also a mixture of antibodies against multiple different epitopes. Even the conventional serum antibodies directed against the same epitope are still composed of heterogeneous antibodies produced by different B cell clones. Therefore, conventional serum antibodies are also called polyclonal antibodies, or polyclonal antibodies for short.
Monoclonal antibody (monoclonal antibody) is a highly uniform antibody produced by a single B cell clone and only directed against a specific epitope. It is usually prepared by hybridoma technology—hybridoma antibody technology is based on cell fusion technology, combining B cells with the ability to secrete specific antibodies and myeloma cells with infinite growth capacity into B-cell hybridomas . This hybridoma cell has the characteristics of a parent cell. It can proliferate indefinitely and immortally in vitro like myeloma cells, and it can synthesize and secrete specific antibodies like splenic lymphocytes. Through cloning, a monoclonal line derived from a single hybridoma cell, that is, a hybridoma cell line, can be obtained. The antibodies it produces are highly homogenous antibodies against the same antigenic determinant, that is, monoclonal antibodies.
Antibodies exist as one or more Y-shaped monomers (ie, monoclonal antibodies or polyclonal antibodies). Each Y-shaped monomer is composed of 4 polypeptide chains, including two identical heavy chains and two identical light chains. Light chain and heavy chain are named according to their molecular weight. The top of the Y-shaped structure is the variable region, which is the antigen binding site. (Excerpt from Detai Bio-Monoclonal Antibody Concept)
Antibody structure
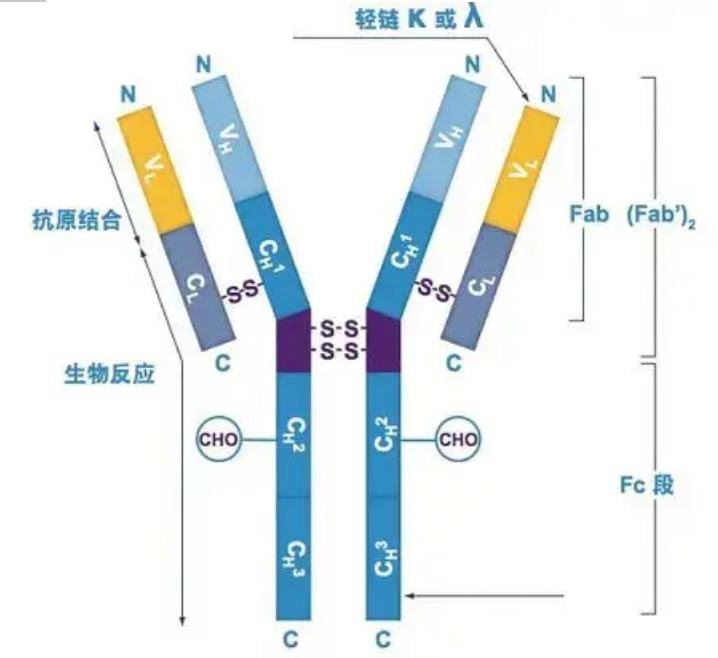 Heavy chain
Heavy chain
There are five types of mammalian Ig heavy chains, named with the Greek letters α, δ, ε, γ, and μ. The corresponding antibodies are called IgA, IgD, IgE, IgG, and IgM. Different heavy chains differ in size and composition. α and γ contain approximately 450 amino acids, while μ and ε contain approximately 550 amino acids.
Each heavy chain has two regions: the constant region and the variable region. All antibodies of the same type have the same constant region, but there are differences between antibodies of different types. The constant regions of the heavy chains γ, α, and δ are composed of three Ig domains in tandem, with a hinge region to increase its flexibility; the constant regions of the heavy chains μ and ε are composed of 4 Ig domains. The variable region of the heavy chain of the antibody produced by different B cells is different, but the variable region of the antibody produced by the same B cell or cell clone is the same, and the variable region of each heavy chain is about 110 amino acids in length. , And form a single Ig domain.
Light chain
There are only two types of light chains in mammals: lambda type and kappa type. Each light chain has two linked domains: a constant region and a variable region. The length of the light chain is about 211~217 amino acids. The two light chains contained in each antibody are always the same. For mammals, the light chain in each antibody has only one type: kappa or lambda. In some lower vertebrates, such as cartilaginous fishes (cartilage fishes) and bony fishes, other types of light chains such as the iota (iota) type are also found.
Fab and Fc segments
The Fc segment can be directly combined with enzymes or fluorescent dyes to label antibodies. It is the part where the antibody rivets on the plate during the ELISA process, and it is also the part where the second antibody is recognized and bound in immunoprecipitation, immunoblotting and immunohistochemistry. Antibodies can be hydrolyzed into two F(ab) segments and one Fc segment by proteolytic enzymes such as papain, or they can be broken from the hinge region by pepsin and hydrolyzed into one F(ab)2 segment and one Fc segment. IgG antibody fragments are sometimes very useful. Due to the lack of the Fc segment, the F(ab) segment will not precipitate with the antigen, nor will it be captured by immune cells in in vivo studies. Because of the small molecular fragments and lack of cross-linking function (due to the lack of Fc segment), the Fab segment is usually used for radiolabeling in functional studies, and the Fc segment is mainly used as a blocking agent in histochemical staining.
Variable and constant regions
The variable region (V region) is located at 1/5 or 1/4 (containing about 118 amino acid residues) of the H chain near the N-terminus and 1/2 (containing about 108-111 amino acid residues) near the N-terminus of the L chain . Each V region has a peptide ring formed by intra-chain disulfide bonds, and each peptide ring contains approximately 67 to 75 amino acid residues. The composition and arrangement of amino acids in the V region determine the antigen binding specificity of the antibody. Due to the ever-changing types and sequence of amino acids in the V region, many kinds of antibodies with different binding antigen specificities can be formed. The V regions of the L chain and H chain are called VL and VH, respectively. In VL and VH, the amino acid composition and sequence of some local regions have a higher degree of variation. These regions are called hypervariable regions (HVR). The amino acid composition and arrangement of the non-HVR parts in the V region are relatively conservative, which is called the framework region. There are three hypervariable regions in VL, usually located at amino acid residues 24 to 34 and 89 to 97 respectively. The three HVRs of VL and VH are called HVR1, HVR2 and HVR3, respectively. The research and analysis of X-ray crystal diffraction proved that the hypervariable region is indeed the place where the antibody antigen binds, so it is called the complementarity-determining region (CDR). The HVR1, HVR2 and HVR3 of VL and VH can be called CDR1, CDR2 and CDR3 respectively. Generally, CDR3 has a higher degree of hypervariability. The hypervariable region is also the main location where the idiotypic determinants of Ig molecules exist. In most cases, the H chain plays a more important role in binding to the antigen.
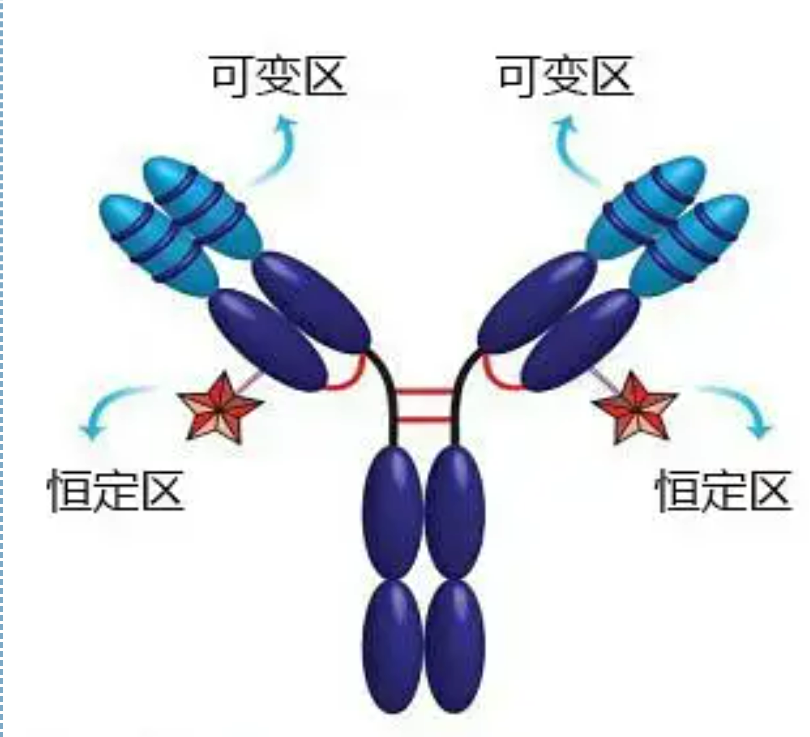 The constant region (C region) is located at 3/4 or 4/5 (approximately from amino acid 119 to the C terminal) of the H chain near the C terminus and 1/2 (contains about 105 amino acid residues) near the C terminus of the L chain. Each functional region of the H chain contains about 110 amino acid residues, and contains a peptide ring composed of 50-60 amino acid residues connected by disulfide bonds. The amino acid composition and arrangement of this region are relatively constant in the same animal Ig isotype L chain and the same type H chain. The same, it can only specifically bind to the corresponding antigen, but the structure of its C region is the same, that is, it has the same antigenicity. The horse anti-human IgG secondary antibody (or anti-antibody) can be combined with the two A combination of antibodies (IgG) against different exotoxins occurs. This is an important basis for preparing secondary antibodies and applying fluorescein, isotopes, enzymes and other labeled antibodies.
The constant region (C region) is located at 3/4 or 4/5 (approximately from amino acid 119 to the C terminal) of the H chain near the C terminus and 1/2 (contains about 105 amino acid residues) near the C terminus of the L chain. Each functional region of the H chain contains about 110 amino acid residues, and contains a peptide ring composed of 50-60 amino acid residues connected by disulfide bonds. The amino acid composition and arrangement of this region are relatively constant in the same animal Ig isotype L chain and the same type H chain. The same, it can only specifically bind to the corresponding antigen, but the structure of its C region is the same, that is, it has the same antigenicity. The horse anti-human IgG secondary antibody (or anti-antibody) can be combined with the two A combination of antibodies (IgG) against different exotoxins occurs. This is an important basis for preparing secondary antibodies and applying fluorescein, isotopes, enzymes and other labeled antibodies.
Related Products:
Cell Direct RT-qPCR kit
Post time: Sep-30-2021








