Establish PCR experiment SOP to standardize the behavior of experiment personnel.

The experimenter strictly abides by the operating procedures, and minimizes the PCR pollution that may be caused by human factors or prevents the occurrence of pollution. In addition, the experimenter should have the corresponding professional knowledge and skills, including proficiency in operating related equipment, clarifying the entire work process, mastering the treatment methods of contamination and laboratory quality control methods, and being able to correctly interpret the test results.
Establish a standard PCR laboratory.

PCR laboratory is divided into four areas in principle, namely reagent preparation area, sample processing area, amplification area, and amplification product analysis area. The first two areas are pre-amplification areas, and the last two areas are post-amplification areas. The pre-amplification zone and the post-amplification zone should be strictly separated. Experimental materials, reagents, recording paper, pens, cleaning materials, etc., can only flow from the pre-amplification area to the post-amplification area, that is, from reagent preparation area → sample processing area → amplification area → amplification product analysis area, and must not flow backwards . The airflow in the laboratory should also flow from the pre-amplification area to the post-amplification area, and not flow backwards. The ideal PCR laboratory design is shown below:
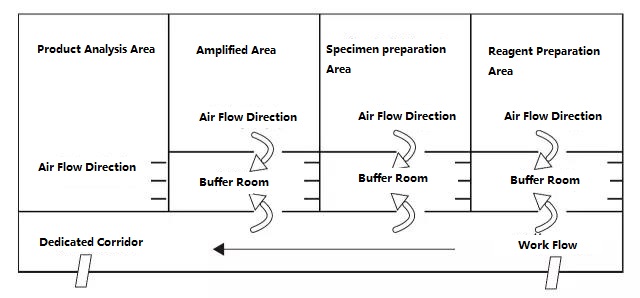
Figure A: Ideal PCR laboratory setup mode with negative pressure in the buffer room
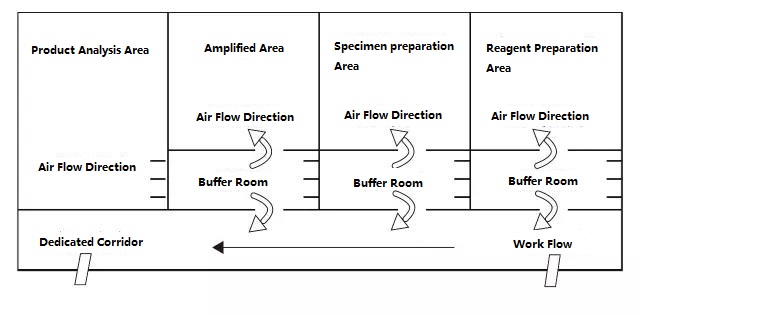
Figure B: Ideal PCR laboratory setup mode with positive pressure in the buffer room
The PCR laboratory setup diagrams given in Figure A and Figure B should be a more ideal setup mode, and the laboratory with conditions can refer to this mode for design. For ordinary laboratories, it is recommended that the PCR amplification area and the product analysis area can be separated, and the opening of the cover should be reduced as much as possible in the sample preparation area and the PCR amplification area. Remember: Products and experimental supplies in the product analysis area are strictly prohibited from being taken to the sample preparation area and PCR amplification area.

If the laboratory only performs PCR detection and identification, it is recommended to use fluorescent quantitative PCR instead of conventional PCR.
Fluorescence quantitative PCR detection results can be collected and analyzed by fluorescence signals, so there is no need to open the lid for electrophoresis after the reaction, which avoids the PCR product contamination caused by the leakage of reaction products to form aerosols. If you increase the number of cap openings during the loading step of gel electrophoresis, aerosol contamination is likely to occur. It is recommended to promote the application of quantitative PCR and gradually replace qualitative PCR.
The UNG anti-PCR product contamination system is used for the PCR reaction.
The system uses dUTP instead of dTTP. After the PCR reaction, all PCR products (DNA fragments) are incorporated with dUTP; in the next round of PCR reaction, the UNG enzyme added to the system is incubated at 37°C for 5 minutes before PCR, which can specifically degrade all DNA fragments containing dUTP, and then perform PCR reaction. This can completely remove the aerosol contamination caused by PCR products. The effect is shown in the figure below:
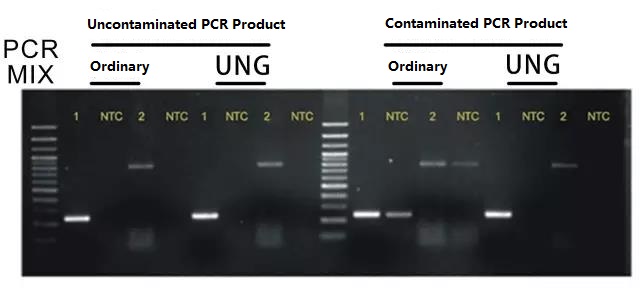
Note: For the direct PCR series, you can choose the series products of the anti-PCR product contamination system of Foregene Suggest
For laboratories that conduct large-scale genotyping testing, it is strongly recommended to use the UNG anti-PCR product contamination system for testing reagents in addition to the construction of reasonable laboratories.
Reminder: The use of this system cannot remove the PCR product contamination that has already been caused. Therefore, the UNG system should be used at the beginning of the relevant test, and the UNG system should be used for PCR amplification, so as to prevent the contamination of PCR products False positive.
It is recommended to use the Direct PCR-UNG system of Foregene when conducting large-scale testing, such as:
Plant Leaf Direct PCR Kit-UNG;
Plant Seed Direct PCR Kit-UNG;
Animal Tissue Direct PCR Kit-UNG;
Mouse Tail Direct PCR Kit-UNG;
Zebra Fish Direct PCR Kit-UNG。
This series of kits from Foregene can not only perform PCR detection quickly and on a large scale, but also effectively prevent and control PCR product contamination.
Post time: Mar-19-2021








