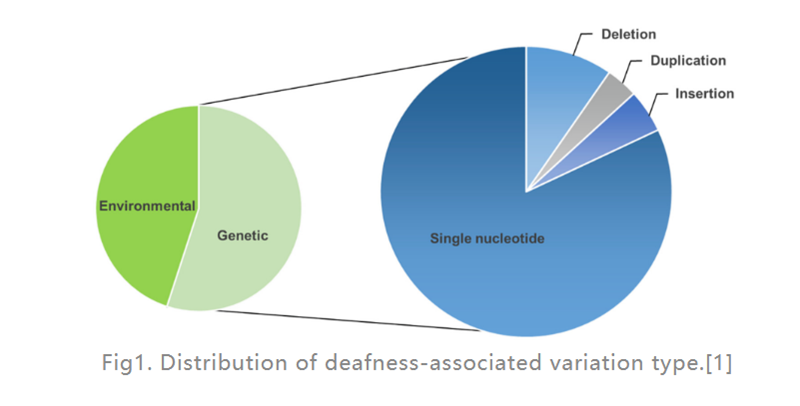
Hearing loss (HL) is the most common sensory disability disease in humans. In developed countries, about 80% of prelingual deafness cases in children are caused by genetic factors. The most common are single-gene defects (as shown in Fig. 1), 124 gene mutations have been found to be associated with nonsyndromic hearing loss in humans, the rest are caused by environmental factors. A cochlear implant (an electronic device placed in the inner ear that provides electrical stimulation directly to the auditory nerve) is by far the most effective option for treating severe HL, while a hearing aid (an external electronic device that converts and amplifies sound waves) can help Patients with moderate HL. However, there are currently no drugs available to treat hereditary HL (GHL). In recent years, gene therapy has received increasing attention as a promising approach to treat inner ear dysfunction.
Fig1. Distribution of deafness-associated variation type.[1]
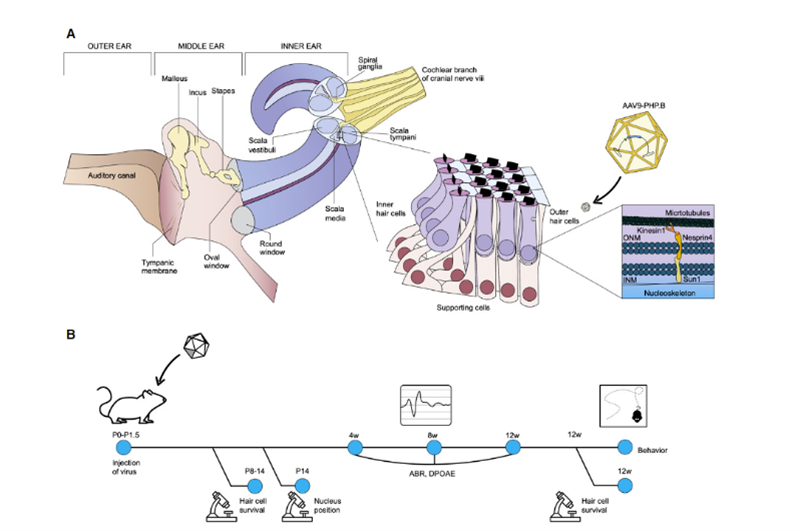
Recently, scientists from the Salk Institute and the University of Sheffield published a research result in Molecular Therapy – Methods & Clinical Development [2], which showed broad application prospects for in vivo gene therapy of hereditary deafness. Uri Manor, assistant research professor at the Salk Institute and director of the Waitt Center for Advanced Biophotonics, said he was born with severe hearing loss and felt that restoring hearing would be a wonderful gift. His previous research found that Eps8 is an actin regulatory protein with actin binding and capping activities; in cochlear hair cells, the protein complex formed by Eps8 with MYO15A, WHIRLIN, GPSM2 and GNAI3 mainly exists in the most The tips of long stereocilia, which together with MYO15A localize BAIAP2L2 at the tips of shorter stereocilia, are required for the maintenance of hair bundles. Therefore, Eps8 can regulate the length of the stereocilia of hair cells, which is essential for normal hearing function; Eps8 deletion or mutation will lead to short stereocilia, which make it unable to properly convert sound into electrical signals for brain perception, which in turn leads to deafness. . At the same time, collaborator Walter Marcotti, a professor at the University of Sheffield, found that hair cells cannot develop normally in the absence of Eps8. In this study, Manor and Marcotti teamed up to investigate whether adding Eps8 to stereociliary cells could restore their function and, in turn, improve hearing in mice. The research team used the adeno-associated virus (AAV) vector Anc80L65 to deliver the coding sequence containing wild-type EPS8 into the cochlea of Eps8-/- newborn P1-P2 mice by round window membrane injection; in mouse cochlear hair cells The function of stereocilia was repaired before they matured; and the repair effect was characterized by imaging technology and measurement of stereocilia. The results showed that Eps8 increased the length of stereocilia and restored hair cell function in low-frequency cells. They also found that, over time, the cells seemed to lose their ability to be rescued by this gene therapy. The implication is that this treatment may need to be administered in utero, as Eps8-/- hair cells may have matured or accumulated damage beyond repair after the mice were born. “Eps8 is a protein with many different functions, and there’s still a lot to explore,” Manor said. Future research will include investigating the effect of Eps8 gene therapy in restoring hearing at different developmental stages, and whether it may be possible to prolong treatment opportunities. Coincidentally, in November 2020, Professor KarenB Avraham of Tel Aviv University in Israel published his results in the journal EMBO Molecular Medicine [3], using an innovative gene therapy technology to create a harmless synthetic adeno-associated virus AAV9-PHP. B, The gene defect in the hair cells of Syne4-/- mice was repaired by injecting a virus carrying the coding sequence of Syne4 into the inner ear of mice, allowing it to enter the hair cells and release the carried genetic material, allowing them to mature and function normally (as in Fig. 2).
Fig2. Schematic representation of inner ear anatomy, with a focus on theorgan of Corti and the cellular function of nesprin-4.
It can be seen that the use of gene therapy to achieve the purpose of treating hereditary diseases at the gene level by inserting, removing or correcting any mutated genes for treatment (that is, controlling the genetic changes in the disease) has a high clinical effect. application prospects. The current gene therapy methods for genetically deficient deafness can be divided into the following categories:
gene replacement
Gene replacement is arguably the most “straightforward” form of gene therapy, based on identifying and replacing a defective gene with a normal or wild-type copy of the gene. First successful inner ear gene therapy study for hearing loss caused by deletion of the vesicular glutamate transporter 3 (VGLUT3) gene; AAV1-mediated delivery of exogenous VGLUT3 overexpression in inner ear hair cells (IHCs) Can result in sustained hearing recovery, partial ribbon synaptic morphology recovery, and convulsive responses [4]. However, in the examples including the two AAV-delivered gene replacements described in the introduction above, it is important to note that the mouse models used for certain types of gene deletion hereditary hearing loss disorders are temporally different from humans, and in P1 mice , the inner ear is in the mature stage of development. In contrast, humans are born with a mature inner ear. This difference prevents possible application of the mouse results to the treatment of human hereditary deafness disorders unless gene therapy is delivered to mature mouse ears.
Gene Editing: CRISPR/Cas9
Compared with “gene replacement”, the development of gene editing technology has brought the dawn of treating genetic diseases from the root. Importantly, the gene editing method makes up for the shortcomings of traditional overexpression gene therapy methods that are not suitable for dominant hereditary deafness diseases, and the problem that the overexpression method does not last long. After Chinese researchers specifically knocked out the Myo6C442Y mutant allele in Myo6WT/C442Y mice using the AAV-SaCas9-KKH-Myo6-g2 gene editing system, and within 5 months of the knockout, mice The auditory function of the model was restored; at the same time, it was also observed that the survival rate of hair cells in the inner ear was improved, the shape of the cilia became regular, and the electrophysiological indicators were corrected [5]. This is the first study in the world to use CRISPR/Cas9 technology for the treatment of hereditary deafness caused by Myo6 gene mutation, and it is an important research progress of gene editing technology for the treatment of hereditary deafness. The clinical translation of treatment provides a solid scientific basis.
Gene therapy delivery methods
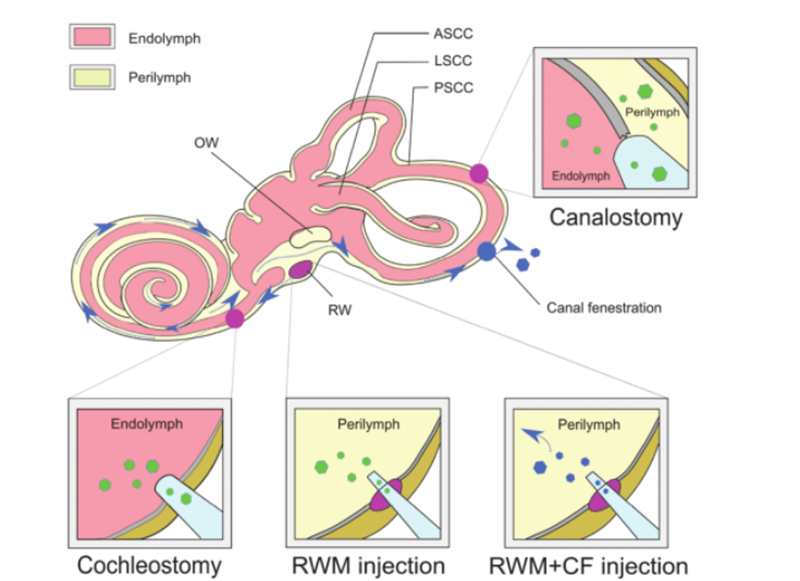
In order for gene therapy to be successful, naked DNA molecules cannot penetrate cells effectively due to their hydrophilicity and negative charge of phosphate groups, and to ensure the integrity of the supplemented nucleic acid molecules, a safe and effective method must be selected. The supplemented DNA is delivered to the target cell or tissue. AAV is widely used as a delivery vehicle for disease treatment due to its high infectious effect, low immunogenicity, and broad tropism to various tissue types. At present, a large body of research work has determined the tropism of different subtypes of AAV relative to different cell types in the mouse cochlea. Using AAV delivery characteristics combined with cell-specific promoters can achieve cell-specific expression, which can reduce off-target effects. In addition, as an alternative to traditional AAV vectors, new synthetic AAV vectors are constantly being developed and show superior transduction ability in the inner ear, of which AAV2/Anc80L65 is the most widely used. Non-viral delivery methods can be further divided into physical methods (microinjection and electroporation) and chemical methods (lipid-based, polymer-based, and gold nanoparticles). Both approaches have been used in the treatment of hereditary deafness disorders and have shown different advantages and limitations. In addition to the delivery vehicle for gene therapy as a vehicle, different approaches for in vivo gene administration can be employed based on different target cell types, routes of administration, and therapeutic efficacy. The intricate structure of the inner ear makes it difficult to reach target cells and the distribution of genome editing agents is slow. The membranous labyrinth is located within the bony labyrinth of the temporal bone and includes the cochlear duct, semicircular duct, utricle, and balloon. Its relative isolation, minimal lymphatic circulation, and separation from the blood by a blood-maze barrier limit the effective systemic delivery of therapeutics to neonatal mice only. To obtain viral titers suitable for gene therapy, direct local injection of viral vectors into the inner ear is necessary. Established routes of injection include [6]: (1) round window membrane (RWM), (2) tracheostomy, (3) endolymphatic or perilymphatic cochleostomy, (4) round window membrane plus Tube fenestration (CF) (as in Fig. 3).
Fig3. Inner ear delivery of gene therapy.
Although many advances have been made in gene therapy, based on clinical translational goals, more work needs to be done before gene therapy can become a first-line treatment option for patients with genetic diseases, especially in the development of safe and effective vectors and delivery method. But we believe that in the near future, these types of treatments will become a staple of personalized therapy and will have a hugely positive impact on the lives of people with genetic disorders and their families.
Foregene has also launched a high-throughput screening kit for targeted genes, which is fast and can perform reverse transcription and qPCR reactions without RNA extraction.
Product Links
Cell Direct RT-qPCR kit—Taqman/SYBR GREEN I
For more product information, please contact:
Post time: Sep-02-2022