At the Vaccine and Health Conference, experts called for "everyone should pay attention to mRNA vaccines, which provide human beings with unlimited thinking." So what exactly is an mRNA vaccine? How was it discovered and what is its application value? Can it resist the COVID-19 raging around the world? Has my country successfully developed an mRNA vaccine? Today, let's learn about the past and present of mRNA vaccines.
01
What is mRNA in mRNA vaccines?
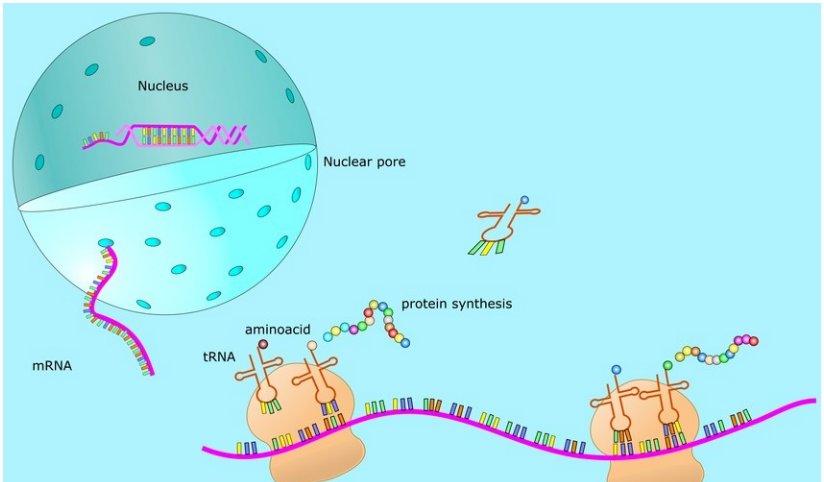
mRNA (Messenger RNA), that is, messenger RNA, is a type of single-stranded RNA that is transcribed from a strand of DNA as a template and carries genetic information that can guide protein synthesis. In layman's terms, mRNA replicates the genetic information of one strand of double-stranded DNA in the nucleus, and then leaves the nucleus to produce proteins in the cytoplasm. In the cytoplasm, ribosomes move along the mRNA, read its base sequence, and translate it into its corresponding amino acid, ultimately forming a protein (Figure 1).
Figure 1 mRNA working process
02
What is an mRNA vaccine and what makes it unique?
mRNA vaccines introduce mRNA encoding disease-specific antigens into the body, and use the host cell's protein synthesis mechanism to generate antigens, thereby triggering an immune response. Usually, mRNA sequences of specific antigens can be constructed according to different diseases, packaged and transported into cells by novel lipid nanocarrier particles, and then the mRNA sequences of human ribosomes are used to translate the mRNA sequences to produce disease antigen proteins, which are recognized by the autoimmune system after secretion to generate an immune response , so as to achieve the role of disease prevention (Figure 2).
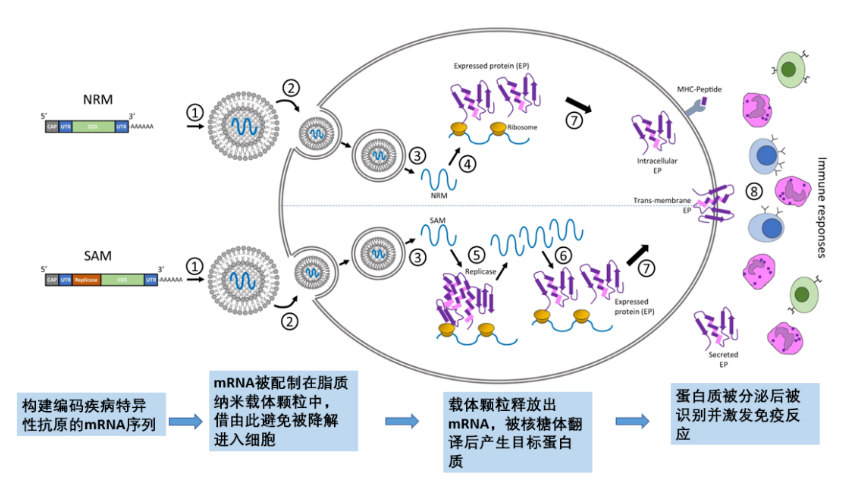 Figure 2. In vivo effect of mRNA vaccine
Figure 2. In vivo effect of mRNA vaccine
So, what is unique about this type of mRNA vaccine compared to traditional vaccines? mRNA vaccines are the most cutting-edge third-generation vaccines, and further research is needed to enhance their stability, regulate their immunogenicity, and develop new delivery technologies.
The first generation of traditional vaccines mainly include inactivated vaccines and live attenuated vaccines, which are the most widely used. Inactivated vaccines refer to first culturing viruses or bacteria, and then inactivating them with heat or chemicals (usually formalin); live attenuated vaccines refer to pathogens that mutate and weaken their toxicity after various treatments. but still retains its immunogenicity. Inoculating it into the body will not cause the occurrence of disease, but the pathogen can grow and multiply in the body, trigger the body's immune response, and play a role in obtaining long-term or life-long protection.
The second generation of new vaccines includes subunit vaccines and recombinant protein vaccines. Subunit vaccine is a vaccine subunit vaccine made of the main protective immunogen components of pathogenic bacteria, that is, through chemical decomposition or controlled proteolysis, the special protein structure of bacteria and viruses is extracted and screened out. Vaccines made of immunologically active fragments; recombinant protein vaccines are antigen recombinant proteins produced in different cell expression systems.
The third generation of cutting-edge vaccines includes DNA vaccines and mRNA vaccines. It is to directly introduce the viral gene fragment (DNA or RNA) encoding a certain antigenic protein into the animal somatic cells (vaccine injection into the human body), and produce the antigenic protein through the protein synthesis system of the host cell, inducing the host to produce immunity to the antigenic protein response in order to achieve the purpose of prevention and treatment of disease. The difference between the two is that DNA is first transcribed into mRNA and then protein is synthesized, while mRNA is directly synthesized.
03
The discovery history and application value of mRNA vaccine
When it comes to mRNA vaccines, we have to mention an outstanding female scientist, Kati Kariko, who has laid a solid scientific research foundation for the advent of mRNA vaccines. She was full of research interest in mRNA while she was studying. In her more than 40 years of scientific research career, she suffered repeated setbacks, did not apply for scientific research funds, and did not have a stable scientific research position, but she has always insisted on mRNA research.
There are three important nodes in the advent of mRNA vaccines.
In the first step, she succeeded in producing the desired mRNA molecule through cell culture, but she encountered a problem in making the mRNA function in the body: after injecting the mRNA into the mouse, it would be swallowed by the mouse's immune system. Then she met Weissman. They used a molecule in tRNA called pseudouridine to make mRNA evade the immune response. ][2].
In the second step, around 2000, Prof. Pieter Cullis studied lipid nanotechnology LNPs for in vivo delivery of siRNA for gene silencing applications [3][4]. Weissman organization Kariko et al. found that LNP is a suitable carrier of mRNA in vivo, and may become a valuable tool for delivering mRNA encoding therapeutic proteins, and subsequently verified in the prevention of Zika virus, HIV and tumors [5] ][6][7][8].
In the third step, in 2010 and 2013, Moderna and BioNTech successively obtained patent licenses related to mRNA synthesis from the University of Pennsylvania for further development. Katalin also became the senior vice president of BioNTech in 2013 to further develop mRNA vaccines.
Today, mRNA vaccines can be used in infectious diseases, tumors, and asthma. In the case of COVID-19 raging around the world, mRNA vaccines may play a role as a vanguard.
04
The application prospect of mRNA vaccine in COVID-19
With the global epidemic of COVID-19, countries are working hard to develop a vaccine to curb the epidemic. As a new type of vaccine, mRNA vaccine has played a leading role in the advent of the new crown epidemic. Many top journals have reported the role of mRNA in the SARS-CoV-2 new coronavirus (Figure 3).
Figure 3 Report on mRNA vaccines to prevent new coronavirus (from NCBI)
First of all, many scientists have reported the research of mRNA vaccine (SARS-CoV-2 mRNA) against the new coronavirus in mice. For example: lipid nanoparticle-encapsulated-nucleoside-modified mRNA (mRNA-LNP) vaccine, a single-dose injection induces strong type 1 CD4+ T and CD8+ T cell responses, long-lived plasma and memory B cell responses, and robust and Sustained neutralizing antibody response. This indicates that mRNA-LNP vaccine is a promising candidate against COVID-19[9][10].
Second, some scientists compared the effects of SARS-CoV-2 mRNA and traditional vaccines. Compared with recombinant protein vaccines: mRNA vaccines are far superior to protein vaccines in germinal center response, Tfh activation, neutralizing antibody production, specific memory B cells, and long-lived plasma cells [11] .
Then, as SARS-CoV-2 mRNA vaccine candidates entered clinical trials, concerns were raised about the short duration of vaccine protection. Scientists have developed a lipid-encapsulated form of a nucleoside-modified mRNA vaccine called mRNA-RBD. A single injection can generate strong neutralizing antibodies and cellular responses, and can almost completely protect model mice infected with 2019-nCoV, with high levels of neutralizing antibodies maintained for at least 6.5 months. These data suggest that a single dose of mRNA-RBD provides long-term protection against SARS-CoV-2 challenge [12].
There are also scientists working to develop new safe and effective vaccines against COVID-19, such as the BNT162b vaccine. Protected macaques from SARS-CoV-2, protected the lower respiratory tract from viral RNA, produced highly potent antibodies, and showed no signs of disease enhancement. Two candidates are currently under evaluation in phase I trials, and evaluation in global phase II/III trials is also underway, and application is just around the corner [13].
05
The status of mRNA vaccine in the world
At present, BioNTech, Moderna and CureVac are known as the world's top three mRNA therapy leaders. Among them, BioNTech and Moderna are at the forefront of the research and development of the new crown vaccine. Moderna has been focusing on the research and development of mRNA-related drugs and vaccines. The COVID-19 phase III trial vaccine mRNA-1273 is the company's fastest-growing project. BioNTech is also a world-leading mRNA drug and vaccine research and development company, with a total of 19 mRNA drugs/vaccines, 7 of which have entered the clinical stage. CureVac has been focusing on the research and development of mRNA drugs/vaccines, and is the first company in the world to establish a GMP-compliant RNA production line, focusing on tumors, infectious diseases and rare diseases.
Related products: RNase Inhibitor
Key words: miRNA vaccine, RNA Isolation, RNA extraction, RNase Inhibitor
References:1. K Karikó, Buckstein M , Ni H , et al. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA[J]. Immunity, 2005, 23(2):165-175.
2. K Karikó, Muramatsu H , Welsh F A , et al. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability[J]. Molecular Therapy, 2008.3. Chonn A , Cullis P R . Recent advances in liposome technologies and their applications for systemic gene delivery[J]. Advanced Drug Delivery Reviews, 1998, 30(1-3):73.4. Kulkarni J A , Witzigmann D , Chen S , et al. Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics[J]. Accounts of Chemical Research, 2019, 52(9).5. Kariko, Katalin, Madden, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes[J]. Journal of Controlled Release Official Journal of the Controlled Release Society, 2015.6. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination[J]. Nature, 2017, 543(7644):248-251.7. Pardi N , Secreto A J , Shan X , et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge[J]. Nature Communications, 2017, 8:14630.8. Stadler C R , B?Hr-Mahmud H , Celik L , et al. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies[J]. Nature Medicine, 2017.9. NN Zhang, Li X F , Deng Y Q , et al. A Thermostable mRNA Vaccine against COVID-19[J]. Cell, 2020.10. D Laczkó, Hogan M J , Toulmin S A , et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice - ScienceDirect[J]. 2020.11. Lederer K , Castao D , Atria D G , et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation[J]. Immunity, 2020, 53(6):1281-1295.e5.12. Huang Q , Ji K , Tian S , et al. A single-dose mRNA vaccine provides a long-term protection for hACE2 transgenic mice from SARS-CoV-2[J]. Nature Communications.13. Vogel A B , Kanevsky I , Ye C , et al. Immunogenic BNT162b vaccines protect rhesus macaques from SARS-CoV-2[J]. Nature, 2021:1-10.
Post time: Jun-20-2022