Foregene-’SARS-CoV-2 Nucleic Acid Detection Kit (Multiplex PCR Fluorescent Probe Method)‘
Since the beginning of the outbreak, Foregene has paid close attention to it, and at the same time urgently organized scientific research forces, and invested the first time in the research and development of the new coronavirus nucleic acid detection kit, based on years of accumulated technical precipitation and experience , develope the SARS-CoV-2 Nucleic Acid Detection Kit (Multiplex PCR Fluorescent Probe Method) at the earliest time.
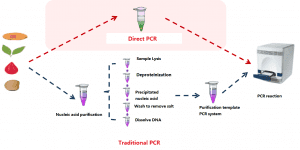
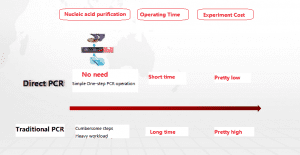
The kit adopts the patented technology of Foregene’s Direct PCR (direct PCR), which eliminates the need for nucleic acid purification of the sample. After simple nucleic acid release treatment, it can be added to the reaction solution for machine detection. The operation is simple and quick, only takes 40 to 60 minutes to obtain 96 test results, which effectively reduces the labor load of the operators. The application scenarios are flexible. Only one type of fluorescent quantitative PCR instrument is required to provide the most effective result judgment for epidemic prevention and control.
Advantages of direct PCR compared to traditional PCR
In addition to viral pneumonia, bacterial pneumonia is also an infectious disease that cannot be ignored.
In the treatment of respiratory tract infections, the biggest difficulty faced by emergency doctors is that they cannot obtain pathogenic data in a short time. The lag of the examination results makes clinicians have to seek help from broad-spectrum antibiotics (which can affect a variety of Bacteria), antibiotics will promote the production of multi-drug resistant bacteria.
In the face of this practical problem, Foregene has developed a lower respiratory tract pathogen detection kit (multiplex fluorescent PCR method).
The kit also does not require nucleic acid purification of patient samples. It uses a combination of direct PCR and multiplex PCR to detect Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and influenza addiction in sputum or bronchial lavage fluid in about 1 hour. Blood bacteria and other 15 common clinically common lower respiratory tract pathogens can be effectively distinguished between normal bacteria and pathogenic bacteria. We believe it will be expected to be an effective tool to assist clinicians in the precise medication use.
As one of the domestic companies in the field of molecular diagnostics, in the face of the epidemic, the R&D personnel and production employees of Foegene sacrificed the time to reunite with their families during the Spring Festival holiday. They gathered together, stuck to their posts, and worked overtime. Unremitting efforts have been made to promote the prevention and control of the epidemic.
At the most critical moment of the epidemic, we are united to overcome the difficulties, and are willing to provide adequate reagent protection for front-line medical workers to win this “war epidemic” together with the country and the people!
Post time: Jan-29-2020