There are two main types of risks in PCR laboratories: biosafety risks and nucleic acid contamination risks. The former harms people and the environment, and the latter affects the results of PCR tests. This article is about the PCR laboratory risk monitoring points and the corresponding risk levels brought to you.
01 Division of the PCR laboratory
1. Molecular biology testing laboratory
According to the requirements of Article 1.1 of the Basic Setting Standards for Clinical Gene Amplification Testing Laboratories, PCR laboratories generally consist of four areas: reagent storage and preparation area, specimen preparation area, amplification area, and amplification product analysis area. If the real-time fluorescent PCR method is used, the amplification area and the analysis area can be combined into one area; if the fully automated PCR analyzer is used, the specimen preparation area, the amplification area and the analysis area can be combined into one area.
The “Workbook for New Coronavirus Nucleic Acid Testing in Medical Institutions (Trial Version 2)” stipulates that in principle, laboratories carrying out new coronavirus nucleic acid testing should set up the following areas: reagent storage and preparation area, specimen preparation area, amplification and product analysis area . These three areas should be completely independent of each other in physical space, and there can be no direct communication with air.
2. Sample preparation room
Although samples can be simply prepared in the specimen preparation area, a special sample preparation room is still required when dealing with complex samples and a large number of samples. The sample preparation room has a high risk of biological safety and nucleic acid contamination.
3. Waste treatment room
Improper waste treatment will also bring huge risks of biosafety and nucleic acid contamination to the laboratory. Therefore, the waste treatment room needs to be regularly monitored.
02 Risk monitoring points in PCR laboratories
Separate laboratories are divided into sample preparation room, reagent storage and preparation area, specimen preparation area, amplification and product analysis area, and waste treatment room.
According to the type of sampling site, it is divided into surface, instrument, sample, environmental monitoring and pipette.
The risk level ranges from low to high from one star★ to three stars★★★.
1. Sample preparation room:
It is used for the registration, preparation and inactivation of samples, and the biological safety risk is the highest. Because the samples are not extracted and amplified, except for the pipettes that frequently come into contact with the samples, the risk of nucleic acid contamination in other parts is low.
1-4 Sampling at monitoring points
5-8 Sampling at monitoring point
9-12 monitoring point sampling
1. Reagent storage and preparation area:
It is used for the preparation of storage reagents, the dispensing of reagents and the preparation of the amplification reaction mixture, as well as the storage and preparation of consumables such as centrifuge tubes and pipette tips. There is no direct contact with samples and no positive nucleic acid in this area, so the biosafety risk and nucleic acid contamination risk are low.
13-16 Sampling at monitoring points
17-22 Sampling at monitoring
3. Specimen preparation areang points
It is used to open the transfer barrel, inactivate the specimen (when applicable), extract nucleic acid and add it to the amplification reaction tube, etc. This area may involve the processing and opening of samples, the biological safety risk is high, and nucleic acid extraction is carried out, and the risk of nucleic acid contamination is medium to high.
29 Sampling at monitoring points
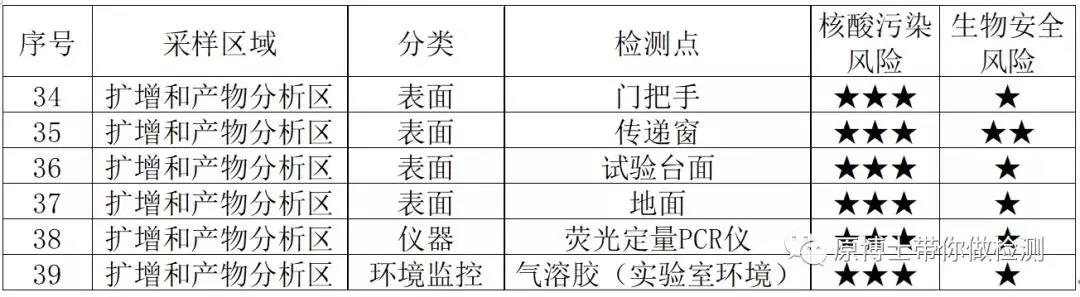
4. Amplification and product analysis area:
Used for nucleic acid amplification. This zone does not involve sample processing, and the biological safety risk is low. The nucleic acid amplification is mainly in this zone, and the risk of nucleic acid contamination is the highest.
38 Sampling at monitoring points
5. Waste treatment room:
Used for high pressure processing of samples. The biological safety risks involved in the processing of samples in this area are relatively high. The nucleic acid amplification products are recommended to be treated as medical waste. High pressure is not recommended, and the risk of nucleic acid contamination is low.
43-44 Sampling at monitoring points
03 Implement
This time we listed 44 monitoring points. It is estimated that many people have to ask, do they need to do so many points? Yes, do it all! I suggest that you first conduct a risk assessment of your own laboratory, which can be done according to the risk from high to low, you can also monitor the same type of samples together, or you can develop a sampling plan for regular monitoring. In short, each laboratory can make its own implementation plan based on its own situation. The biggest risk for testing laboratories is to ignore the risks.
Post time: Jul-03-2021