Real Time PCR Easyᵀᴹ-Taqman
Kit Descriptions
The 2X Real PCR EasyTM Mix-Taqman provided by the Real Time PCR EasyTM-Taqman kit is a new premix system that uses specific fluorescent probes for Real Time PCR amplification reactions, which can greatly improve product specificity and reaction sensitivity. ROX is provided as internal control dye.
2X Real PCR EasyTM Mix-Taqman contains Foregene’s unique hot-start Taq DNA Polymerase. Compared with ordinary Taq enzymes, it has the advantages of high amplification efficiency, strong specific amplification ability and low mismatch rate. It can reduce non-specific amplification and improve the accuracy of PCR.
Specifications
|
Real Time PCR EasyTM-Taqman |
||||
|
Kit composition (20μl system) |
QP-01021 |
QP-01022 |
QP-01023 |
QP-01024 |
|
200T |
500T |
1000T |
2000T |
|
|
2× Real PCR EasyTM Mix-Taqman |
1 ml × 2 |
1.7 ml × 3 |
1.7 ml × 6 |
1.7 ml × 12 |
|
20× ROX Reference Dye |
200 μl |
0.5 ml |
1 ml |
1 ml × 2 |
|
DNase-Free ddH2O |
1.7 ml |
1.7 ml × 2 |
10 ml |
20 ml |
|
Instruction |
1 |
1 |
1 |
1 |
Features&advantages
■ Simple—2X PCR Mix to reduce experimental error and operation time
■ Specific—optimized buffer and hot-start Taq enzyme can prevent non-specific amplification and primer dimer formation
■ High sensitivity—can detect low copies of template
■ Good versatility—compatible with most real-time quantitative PCR instruments
Kit application
qPCR analysis
Work flow
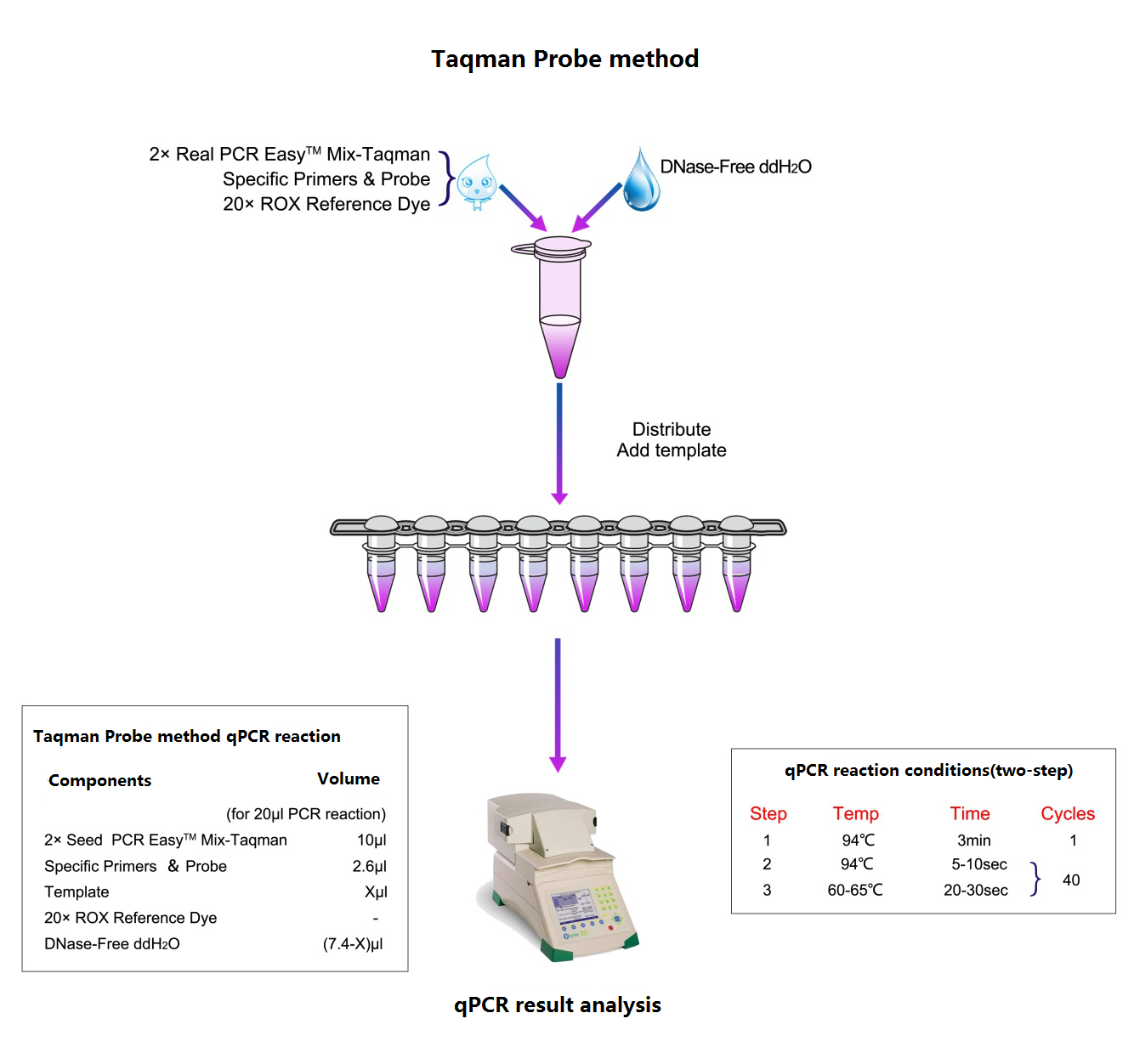
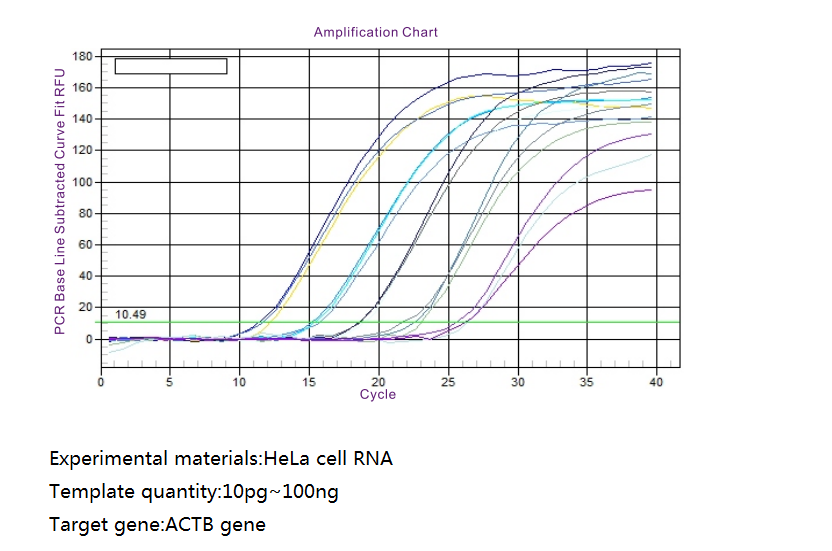
Graphic
Storage and shelf life
This kit should be stored away from light and should be stored at -20℃. If used frequently, it can also be stored at 4℃ for a short period of time (10 days).
No amplification signals
1.The Taq DNA Polymerase in the kit loses its activity due to improper storage or expiration of the kit.
Recommendation: Confirm the storage conditions of the kit; re-add an appropriate amount of Taq DNA Polymerase to the PCR system or purchase a new Real Time PCR Kit for related experiments.
2.There are a lot of inhibitors of Taq DNA Polymerase in the DNA template.
Suggestion: Repurify the template or reduce the amount of template used.
3.The Mg2+ concentration is not suitable.
Recommendation: The Mg2+ concentration of the2× Real PCR Mix we provide is 3.5mM. However, for some special primers and templates, the Mg2+ concentration may be higher. Therefore, you can directly add MgCl2 to optimize the Mg2+ concentration. It is recommended to increase the Mg2+ 0.5mM each time for optimization.
4.The PCR amplification conditions are not suitable, and the primer sequence or concentration is improper.
Suggestion: confirm the correctness of the primer sequence and the primer has not been degraded; if the amplification signal is not good, try to lower the annealing temperature and adjust the primer concentration appropriately.
5.The amount of template is too little or too much.
Recommendation: Perform template linearization gradient dilution, and select the template concentration with the best PCR effect for Real Time PCR experiment.
NTC has too high fluorescence value
1.Reagent contamination caused during operation.
Recommendation: Replace with new reagents for Real Time PCR experiments.
2.Contamination occurred during the preparation of the PCR reaction system.
Recommendation: Take necessary protective measures during operation, such as: wearing latex gloves, using a pipette tip with a filter, etc.
3.The primers are degraded, and the degradation of the primers will cause non-specific amplification.
Suggestion: Use SDS-PAGE electrophoresis to detect whether the primers are degraded, and replace them with new primers for Real Time PCR experiments.
Primer dimer or non-specific amplification
1.The Mg2+ concentration is not suitable.
Recommendation: The Mg2+ concentration of the 2× Real PCR EasyTM Mix we provide is 3.5 mM. However, for some special primers and templates, the Mg2+ concentration may be higher. Therefore, you can directly add MgCl2 to optimize the Mg2+ concentration. It is recommended to increase the Mg2+ 0.5mM each time for optimization.
2.The PCR annealing temperature is too low.
Suggestion: Increase the PCR annealing temperature by 1℃ or 2℃ each time.
3.The PCR product is too long.
Recommendation: The length of the Real Time PCR product should be between 100-150bp, not more than 500bp.
4.The primers are degraded, and the degradation of the primers will lead to the appearance of specific amplification.
Suggestion: Use SDS-PAGE electrophoresis to detect whether the primers are degraded, and replace them with new primers for Real Time PCR experiments.
5.The PCR system is improper, or the system is too small.
Suggestion: The PCR reaction system is too small will cause the detection accuracy to decrease. It is best to use the reaction system recommended by the quantitative PCR instrument to re-run the Real Time PCR experiment.
Poor repeatability of quantitative values
1.The instrument is malfunctioning.
Suggestion: There may be errors between each PCR hole of the instrument, resulting in poor reproducibility during temperature management or detection. Please check according to the instructions of the corresponding instrument.
2.The sample purity is not good.
Recommendation: Impure samples will lead to poor reproducibility of the experiment, which includes the purity of the template and primers. It is best to repurify the template, and the primers are best purified by SDS-PAGE.
3.The PCR system preparation and storage time is too long.
Suggestion: Use the Real Time PCR system for PCR experiment immediately after preparation, and don’t leave it aside for too long.
4.The PCR amplification conditions are not suitable, and the primer sequence or concentration is improper.
Suggestion: confirm the correctness of the primer sequence and the primer has not been degraded; if the amplification signal is not good, try to lower the annealing temperature and adjust the primer concentration appropriately.
5.The PCR system is improper, or the system is too small.
Suggestion: The PCR reaction system is too small will cause the detection accuracy to decrease. It is best to use the reaction system recommended by the quantitative PCR instrument to re-run the Real Time PCR experiment.