What is an mRNA vaccine
The mRNA vaccine transfers RNA to the cells of the body to express and produce protein antigens after relevant modifications in vitro, thereby leading the body to produce an immune response against the antigen, thereby expanding the body's immune capacity [1,3].
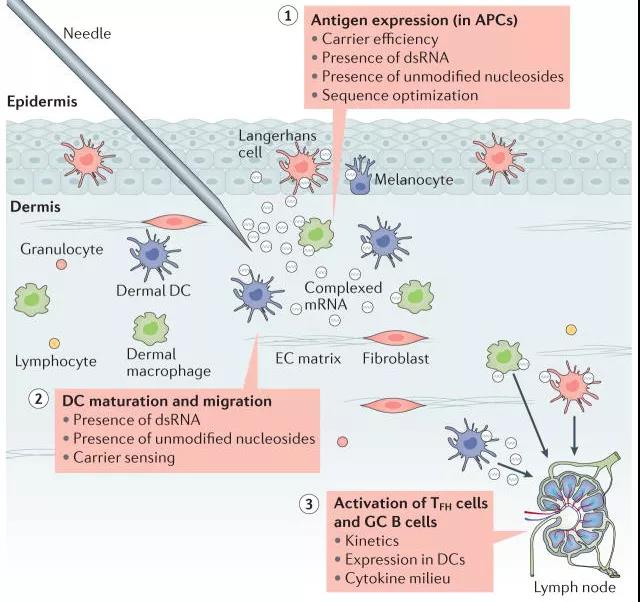
Figure 1: Schematic diagram of the effect of direct injection of mRNA vaccine [2]
Classification of mRNA vaccines
mRNA vaccines are divided into two types: nonreplicating mRNA and self-amplifying mRNA: self-amplifying mRNA not only encodes the target antigen, but also encodes the replication that enables intracellular RNA amplification and protein expression mechanism. Non-replicating mRNA vaccines only encode target antigens and contain 5'and 3'untranslated regions (UTR). They provide comprehensive stimulation of adaptability and innate immunity, namely in situ antigen expression and danger signal transmission, and have the following applications Features [2,3]
●Can provide comprehensive stimulation of adaptability and innate immunity, namely in situ antigen expression and danger signal transmission
●Can induce a "balanced" immune response, including humoral and cellular effectors and immune memory
●Can combine different antigens without increasing the complexity of vaccine formulation
●Continuous improvement of immune potential can be achieved through repeated vaccination, and there is no or little immune response to the carrier
●Heat stable mRNA vaccines can simplify the transportation and storage of vaccines
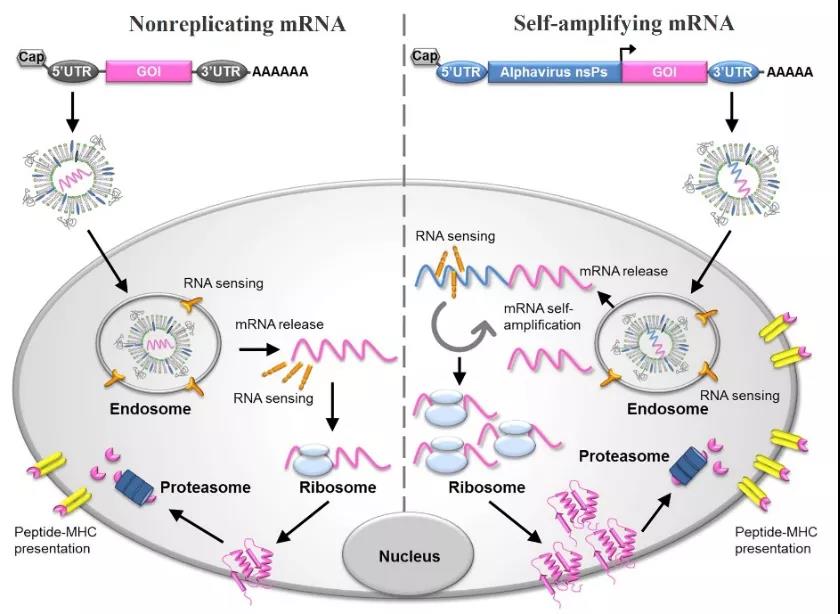
Figure 2: Schematic diagram of mRNA vaccine and its antigen expression mechanism [4]
Features of mRNA vaccines
Compared with traditional vaccines, mRNA vaccines have simple production processes, fast development speeds, no need for cell culture, and low cost. Compared with DNA vaccines, mRNA vaccines do not need to enter the nucleus and there is no risk of integration into the host genome. The half-life can be adjusted by modification.
Table 1: Advantages and disadvantages of mRNA vaccines
|
|
Advantage |
Shortcoming |
|
mRNA vaccine |
Rapid research and development, vaccine production only takes 40 days |
Trigger an unnecessary immune response
|
|
mRNA instability under physiological conditions, easy to degrade |
Will not integrate into the genome to avoid possible therapeutic mutations
|
|
|
No need for any nuclear localization signal, transcription |
The effectiveness of the safety nuclear remains to be verified
|
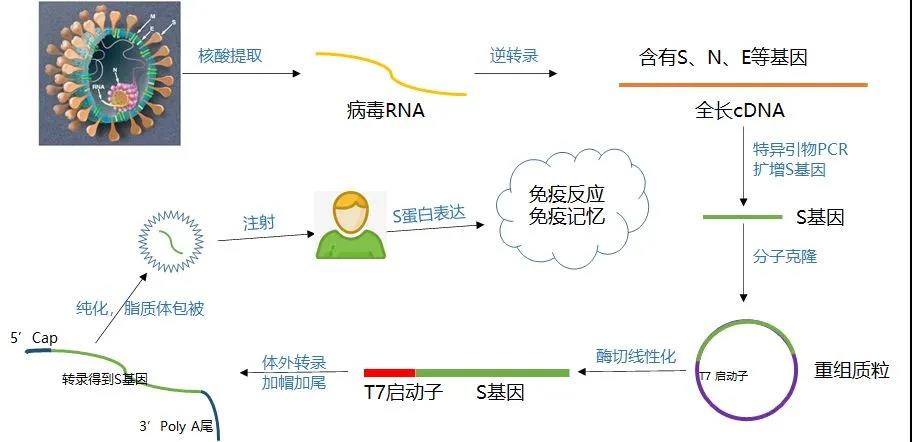
Figure 3: Flow chart of mRNA vaccine production and preparation [4]
Foregene Viral RNA Isolation kit
RT-qPCR Easy (One Step)
Improved strategies for the preparation of mRNA vaccines
Due to the poor stability of mRNA itself, easy degradation by nucleases in tissues, low cell entry efficiency, and low translation efficiency, these defects limit the application of mRNA vaccines. Translation efficiency also plays a very critical role. Delivery vehicles can be divided into viral vectors and non-viral vectors (including liposomes, non-liposomes, viruses, nanoparticles, etc.). Therefore, relevant improvement measures are needed. The following is a pharmacological improvement strategy for mRNA preparation [2]
1 Synthesize cap analogs or use capping enzymes to stabilize mRNA and increase protein translation by binding to eukaryotic translation initiation factor 4E (EIF4E)
2 Adjust the elements in the 5′-untranslated region (UTR) and 3′-UTR to stabilize mRNA and increase protein translation
3 Adding Poly(A) tail can stabilize mRNA and increase protein translation
4 Modified nucleosides to reduce innate immune activation and increase translation
5 Treatment with RNase III and fast protein liquid chromatography (FPLC) purification can reduce immune activation and increase translation
6 Optimize sequences or codons to increase translation
7 Co-delivery of translation initiation factors and other methods to change translation and immunogenicity
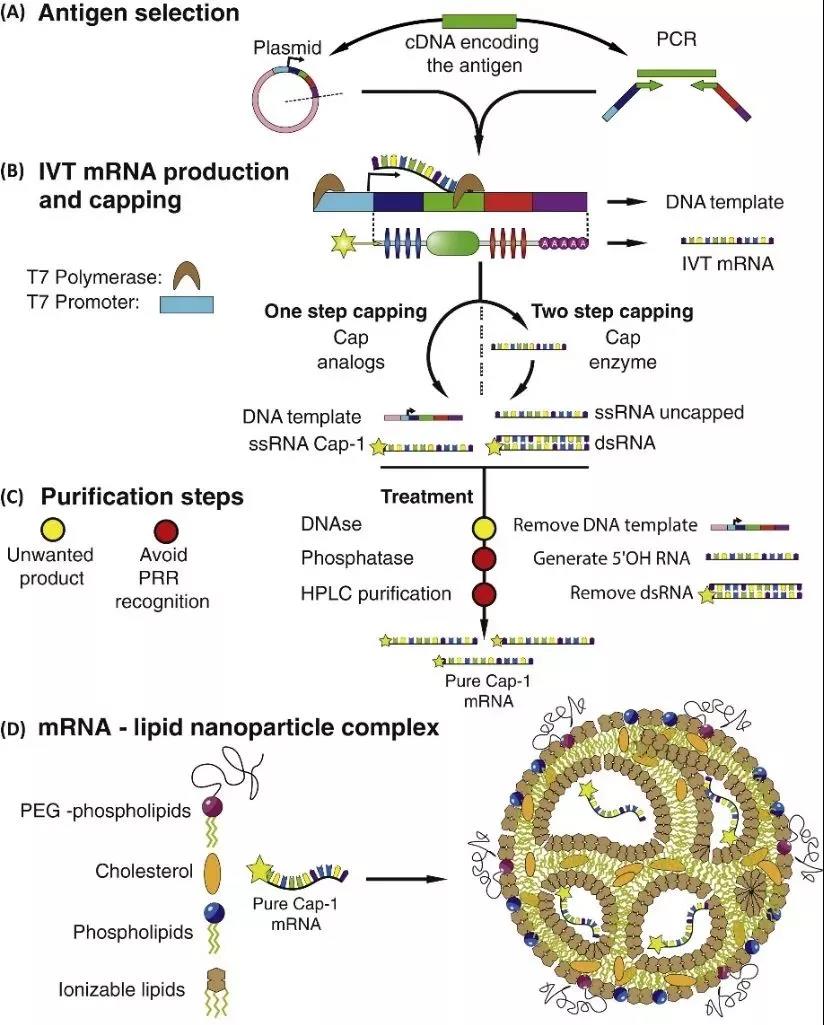
Figure 4: In vitro transcription (IVT) mRNA production and assembly process [5]
Large-scale preparation of plasmid DNA
Plasmid DNA purification mainly removes contaminants such as RNA, open-circle DNA endotoxin, host protein and host nucleic acid, and usually transforms recombinant plasmid into E. coli. E. coli undergoes high-density fermentation, then solid-liquid separation, and collection of E. coli. The E. coli is then subjected to alkaline lysis, centrifugal solid-liquid separation and microfiltration clarification after lysis, ultrafiltration and concentration after clarification, and then chromatographic purification.

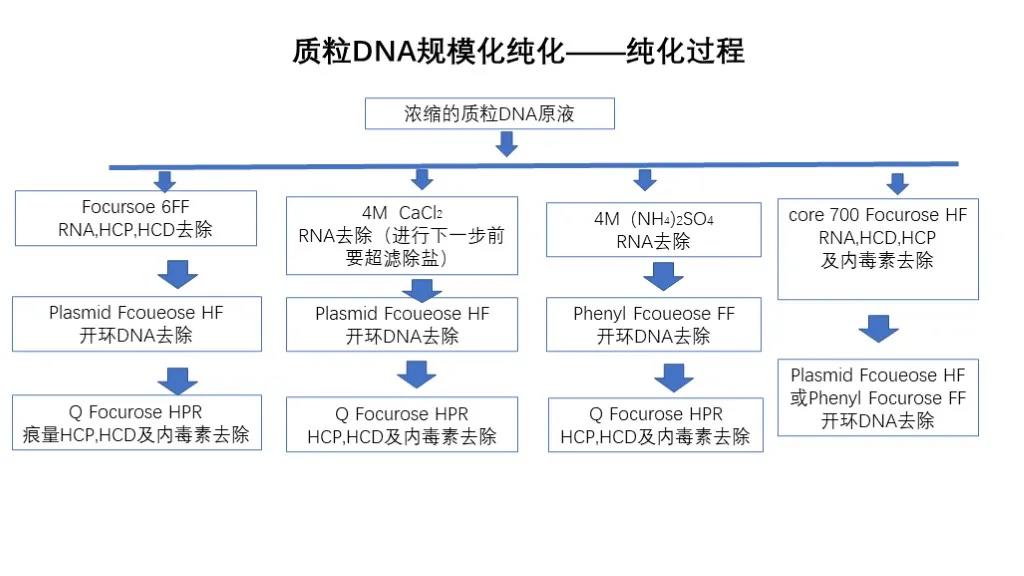
Purification of plasmid DNA:
Foregene General Plasmid Mini Kit
【1】苗鹤凡, 郭勇, 江新香. mRNA疫苗研究进展及挑战[J]. 免疫学杂志, 2016(05):446-449.
【2】Pardi N , Hogan M J , Porter F W , et al. mRNA vaccines — a new era in vaccinology[J]. Nature Reviews Drug Discovery, 2018.
【3】Kramps T., Elbers K. (2017) Introduction to RNA Vaccines. In: Kramps T., Elbers K. (eds) RNA Vaccines. Methods in Molecular Biology, vol 1499. Humana Press, New York, NY.
【4】Maruggi G , Zhang C , Li J , et al. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases[J]. Molecular Therapy, 2019.
【5】Sergio Linares-Fernández, Céline Lacroix, ,Tailoring mRNA Vaccine to Balance Innate/Adaptive Immune Response,Trends in Molecular Medicine,Volume 26, Issue 3,2020,Pages 311-323.
Post time: Aug-05-2021








