With the continuous development of molecular biology technology, the relationship between gene mutations and defects and diseases has gained more and more in-depth understanding. Nucleic acids have attracted much attention because of their great potential for application in the diagnosis and treatment of diseases. Nucleic acid drugs refer to artificially synthesized DNA or RNA fragments with disease treatment functions. Such drugs can directly act on disease-causing target genes or disease-causing target mRNAs, and play a role in treating diseases at the gene level. Compared with traditional small molecule drugs and antibody drugs, nucleic acid drugs can regulate the expression of disease-causing genes from the root, and have the characteristics of “treating the symptoms and curing the root cause”. Nucleic acid drugs also have obvious advantages such as high efficiency, low toxicity, and high specificity. Since the first nucleic acid drug fomivirsen sodium was launched in 1998, many nucleic acid drugs have been approved for clinical treatment.
The nucleic acid drugs currently on the market globally mainly include antisense nucleic acid (ASO), small interfering RNA (siRNA), and nucleic acid aptamers. Except for nucleic acid aptamers (which may exceed 30 nucleotides), nucleic acid drugs are usually oligonucleotides composed of 12 to 30 nucleotides, also known as oligonucleotide drugs. In addition, miRNAs, ribozymes and deoxyribozymes have also shown great development value in the treatment of various diseases. Nucleic acid drugs have become one of the most promising fields in the research and development of biomedicine today.
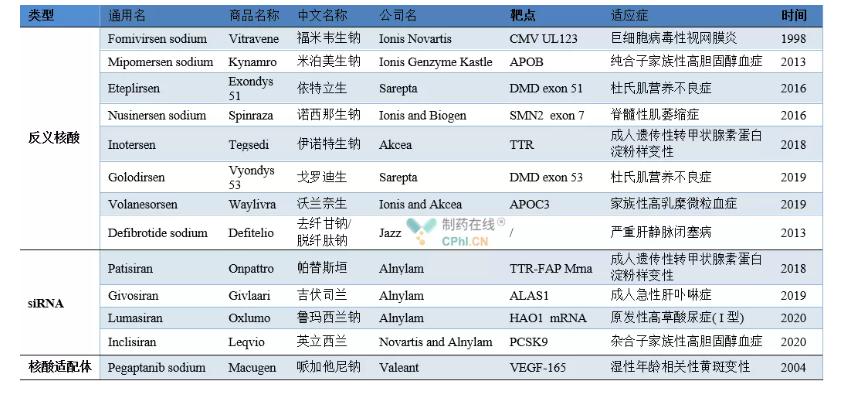
Examples of approved nucleic acid drugs
Antisense nucleic acid
Antisense technology is a new drug development technology based on the principle of Watson-Crick base complementation, using specific complementary DNA or RNA fragments artificially synthesized or synthesized by the organism to specifically regulate the expression of target genes. The antisense nucleic acid has a base sequence complementary to the target RNA and can specifically bind to it. Antisense nucleic acids generally include antisense DNA, antisense RNA and ribozymes. Among them, due to the characteristics of high stability and low cost of antisense DNA, antisense DNA occupies a dominant position in the current research and application of antisense nucleic acid drugs.
Fomivirsen sodium (trade name Vitravene) was developed by Ionis Novartis. In August 1998, the FDA approved it for the treatment of cytomegalovirus retinitis in immunocompromised patients (mainly AIDS patients), becoming the first nucleic acid drug to be marketed. Fomivirsen inhibits partial protein expression of CMV by binding to specific mRNA (IE2), thereby regulating the expression of viral genes to achieve therapeutic effects. However, due to the emergence of high-efficiency antiretroviral therapy, which has greatly reduced the number of patients, in 2002 and 2006, Novartis cancelled the market authorization of Fomivirsen drugs in Europe and the United States respectively, and the product has been suspended from the market.
Mipomersen sodium (trade name Kynamro) is an ASO drug developed by the French company Genzyme. In January 2013, the FDA approved it for the treatment of homozygous familial hypercholesterolemia. Mipomersen inhibits the expression of ApoB-100 protein (apolipoprotein) by binding to ApoB-100mRNA, thereby significantly reducing human low-density lipoprotein cholesterol, low-density lipoprotein and other indicators, but due to side effects such as liver toxicity, December 13, 2012 On the same day, EMA also rejected the application for a sales license for the drug.
In September 2016, Eteplirsen (trade name Exon 51) developed by Sarepta for the treatment of Duchenne muscular dystrophy (DMD) was approved by the FDA. DMD patients cannot normally express functional anti-atrophic protein due to mutations in the DMD gene in the body. Eteplirsen specifically binds to exon 51 of the pre-messenger RNA (Pre-mRNA) of the protein, removes exon 51, and restores some downstream genes The normal expression of, transcription and translation to obtain part of dystrophin, so as to achieve the therapeutic effect.
Nusinersen is an ASO drug developed by Spinraza for the treatment of spinal muscular atrophy and was approved by the FDA on December 23, 2016. In 2018, Inotesen developed by Tegsedi for the treatment of adult hereditary transthyretin amyloidosis was approved by the FDA. In 2019, Golodirsen, developed by Sarepta for the treatment of Duchenne muscular dystrophy, was approved by the FDA. It has the same mechanism of action as Eteplirsen, and its site of action becomes exon 53. In the same year, Volanesorsen, jointly developed by Ionisand Akcea for the treatment of familial hyperchylomicronemia, was approved by the European Medicines Agency (EMA). Volanesorsen regulates triglyceride metabolism by inhibiting the production of apolipoprotein C-Ⅲ, but it also has the side effect of lowering platelet levels.
Defibrotide is an oligonucleotide mixture with plasmin properties developed by Jazz. It contains 90% DNA single-stranded DNA and 10% DNA double-stranded. It was approved by the EMA in 2013 and subsequently approved by the FDA for the treatment of severe hepatic veins. Occlusive disease. Defibrotide can increase the activity of plasmin, increase the plasminogen activator, promote the up-regulation of thrombomodulin, and reduce the expression of von Willebrand factor and plasminogen activator inhibitors to achieve therapeutic effects
siRNA
siRNA is a small fragment of RNA with a specific length and sequence produced by cutting the target RNA. These siRNAs can specifically induce the degradation of target mRNA and achieve gene silencing effects. Compared with chemical small molecule drugs, the gene silencing effect of siRNA drugs has high specificity and efficiency.
On August 11, 2018, the first siRNA drug patisiran (trade name Onpattro) was approved by the FDA and officially launched. This is one of the major milestones in the development history of RNA interference technology. Patisiran was jointly developed by Alnylam and Genzyme, a subsidiary of Sanofi. It is a siRNA drug for the treatment of hereditary thyroxine-mediated amyloidosis. In 2019, givosiran (trade name Givlaari) was approved by the FDA as the second siRNA drug for the treatment of acute hepatic porphyria in adults. In 2020, Alnylam developed a primary type I drug for the treatment of children and adults. Lumasiran with high oxaluria was approved by the FDA. In December 2020, Inclisiran, jointly developed by Novartis and Alnylam for the treatment of adult hypercholesterolemia or mixed dyslipidemia, was approved by the EMA.
Aptamer
Nucleic acid aptamers are oligonucleotides that can bind to a variety of target molecules such as small organic molecules, DNA, RNA, polypeptides or proteins with high affinity and specificity. Compared with antibodies, nucleic acid aptamers have the characteristics of simple synthesis, lower cost and wide range of targets, and have a wider potential for drug application in disease diagnosis, treatment and prevention.
Pegaptanib is the first nucleic acid aptamer drug developed by Valeant for the treatment of wet age-related macular degeneration and was approved by the FDA in 2004. Subsequently, it was approved by EMA and PMDA in January 2006 and July 2008 and went on the market. Pegaptanib inhibits angiogenesis through the combination of spatial structure and vascular endothelial growth factor to achieve therapeutic effects. Since then, it has encountered competition from similar drugs Lucentis, and its market share has dropped a lot.
Nucleic acid drugs have become a hot spot in the clinical drug and new drug market due to their remarkable curative effect and short development cycle. As an emerging drug, it faces challenges while facing opportunities. Due to its exogenous characteristics, the specificity, stability and effective delivery of nucleic acids have become the main criteria for judging whether oligonucleotides can become highly effective nucleic acid drugs. Off-target effects have always been a key point of nucleic acid drugs that cannot be ignored. However, nucleic acid drugs can affect the expression of disease-causing genes from the root, and can achieve sequence specificity at the single-base level, which has the characteristics of “treating the root cause and treating the symptoms”. In view of the variability of more and more diseases, only genetic treatment can achieve permanent results. With the continuous improvement, perfection and progress of related technologies, nucleic acid drugs represented by antisense nucleic acids, siRNA, and nucleic acid aptamers will surely set off a new wave in disease treatment and the pharmaceutical industry.
References:
[1] Liu Shaojin, Feng Xuejiao, Wang Junshu, Xiao Zhengqiang, Cheng Pingsheng. Market analysis of nucleic acid drugs in my country and countermeasures[J]. Chinese Journal of Biological Engineering, 2021, 41(07): 99-109.
[2] Chen Wenfei, Wu Fuhua, Zhang Zhirong, Sun Xun. Research progress in pharmacology of marketed nucleic acid drugs[J]. Chinese Journal of Pharmaceuticals, 2020, 51(12): 1487-1496.
[3] Wang Jun, Wang Lan, Lu Jiazhen, Huang Zhen. Analysis of the efficacy and research progress of marketed nucleic acid drugs[J]. Chinese Journal of New Drugs, 2019, 28(18): 2217-2224.
About the author: Sha Luo, a Chinese medicine research and development worker, currently works for a large domestic drug research and development company, and is committed to the research and development of new Chinese medicines.
Related products:
Post time: Nov-19-2021